INTRODUCTION
As the elderly population increases, a growing number of patients have lower urinary tract symptom (LUTS)/benign prostatic hyperplasia (BPH) [
1]. The treatment of BPH includes watchful waiting, medical management, and surgical treatment. Medication has been widely used in patients with mild to borderline symptoms. The guidelines of the European Urological Association and the American Urological Association indicate that surgical treatment is necessary when patients have urinary retention, renal insufficiency, recurrent urinary tract infections, bladder stone, or gross hematuria due to an enlarged prostate [
2,
3].
However, most patients with LUTS/BPH we encounter in clinical practice do not fall under the above absolute indications. Surgery is additionally needed when patients do not experience adequate relief from LUTS with conservative or medical treatment [
2]. This relative surgical indication usually reflects the subjective judgment of the surgeon, and/or patientsŌĆÖ degree of compliance and preference for surgery [
3]. In real clinical situations, the decision is not simple. It is sometimes difficult to make a clear decision about the treatment of those who do not have an absolute indication for prostatic surgery. Therefore, the development of objective therapeutic guidance is necessary.
LUTS/BPH is a progressive disease that can eventually cause irreversible changes in bladder function, especially if bladder outlet obstruction (BOO) is already present. Therefore, when BOO is evident in patients with bothersome LUTS, surgical treatment should be considered. A pressure-flow study (PFS) is currently considered the gold standard for diagnosing BOO; however, it is an invasive and time-consuming procedure. Noninvasive or less invasive modalities have not been fully validated for clinical use.
At the Seoul National University Hospital (SNUH), we have a large urodynamic study database that has been constructed over the last 13 years. Based on this, we aimed to develop decision support formulas and nomograms predicting the probabilities of having BOO and requiring BOO-related prostatic surgery in patients with LUTS/BPH. We also attempted to validate these formulas and nomograms in a prospective cohort of patients with LUTS/BPH.
MATERIALS AND METHODS
Patients and Evaluation
We have constructed a urodynamic study database at SNUH starting in 2004. From this large database consisting of 11,437 consecutive patients between October 2004 and May 2014, LUTS/BPH patients aged 45 or over were extracted. LUTS involving the following conditions were excluded to construct pure a LUTS/BPH patient dataset (the development cohort): urethral stricture, bladder stone, genitourinary infection or inflammation, genitourinary malignancy, genitourinary radiation, urinary diversion, or neurogenic bladder. We additionally excluded 309 patients who had previously undergone prostatic surgery. We ultimately retrieved 2,732 patients with pure LUTS/BPH.
Clinical parameters were obtained in routine, real clinical practice settings, with careful history-taking, a physical examination including a digital rectal examination, the International Prostate Symptom Score (IPSS), laboratory tests including urinalysis and serum prostate-specific antigen (PSA) levels, and a voiding diary. After free uroflowmetry (UFM), postvoid residual (PVR) volume was measured using an ultrasound bladder scanner (BVI-3000 BladderScan, Verathon, WA, USA or Bio-Con-500, Mcube Technology, Seoul, Korea) in the supine position. The IPSS storage subscore was the sum of IPSS questions 2, 4, and 7, and the voiding subscore was the sum of IPSS questions 1, 3, 5, and 6. UFM results were accepted only when the voided volume was over 120 mL. Prostatic biopsies were performed to exclude cancer when the serum PSA level was elevated (>4 ng/mL) or when a digital rectal examination was suspicious for prostate cancer. Total prostate volume (TPV) was calculated using the prolate ellipsoid formula (ŽĆ/6├Ś height├Świdth├Ślength) based on transrectal ultrasonography (TRUS) measurements in 3 dimensions. A PFS to determine whether BOO was present was performed in our Urodynamic Suite using a multichannel urodynamic machine (UD-2000 or Solar, MMS, Enschede, the Netherlands), as previously described [
4]. We strictly followed the International Continence Society standards in performing urodynamic studies [
5]. BOO was defined as a BOO index Ōēź40; the BOO index was determined by the formula of detrusor pressure at the maximal flow rate (PdetQmax) ŌĆō2├Śmaximal flow rate (Qmax) in the PFS [
6]. Of the 2,732 patients, any patient who was missing 1 or more of the following clinical variables was excluded: IPSS, voided volume for UFM of at least 120 mL, PVR volume, TRUS-measured TPV, PFS, and information regarding surgery.
The electronic medical records of all patients were thoroughly reviewed to exclude any patients who fulfilled the exclusion criteria. Ultimately, a database of pure LUTS/BPH patients with complete clinical variables was obtained as the development cohort for developing both formulas (for having BOO and for undergoing prostatic surgery). The need for prostatic surgery was based on the clinical judgment of urologists, not the actual performance of surgery. Therefore, if patients did not agree to undergo prostatic surgery when clinicians recommended it, we still classified these patients as requiring prostatic surgery. The prostatic surgery techniques for LUTS/BPH included open prostatectomy, transurethral resection of the prostate, transurethral incision of the prostate, laser photoselective vaporization of the prostate, and holmium laser enucleation of the prostate.
Separately from the development cohort, we prospectively enrolled consecutive LUTS/BPH patients with the same inclusion and exclusion criteria between June 2014 and December 2015 at SNUH to construct an internal validation cohort. This study was approved by the Institutional Review Board of SNUH (approval number: H-1406-119-591).
Statistical Analysis
Causal Bayesian network (CBN) analysis was performed to identify clinically relevant variables related to BOO and prostatic surgery. Using ovals to represent variables and arcs for their plausible causal relationships, CBN visually presents first and second-degree relationships among the nomograms of clinical parameters for BOO and prostatic surgery. We used Banjo ver. 2.2.0 (Duke University, Durham, NC, USA; noncommercially available at
https://users.cs.duke.edu/~amink/software/banjo/) [
7,
8] to analyze the collected data and to develop the best-fitting CBN model. The CBN model identified the most relevant clinical variables and predicted the probability of a subject having BOO and the probability of a subject requiring prostatic surgery. We additionally used multivariate logistic regression analysis incorporating the relevant clinical variables to build formulas to calculate the probabilities of having BOO and requiring prostatic surgery.
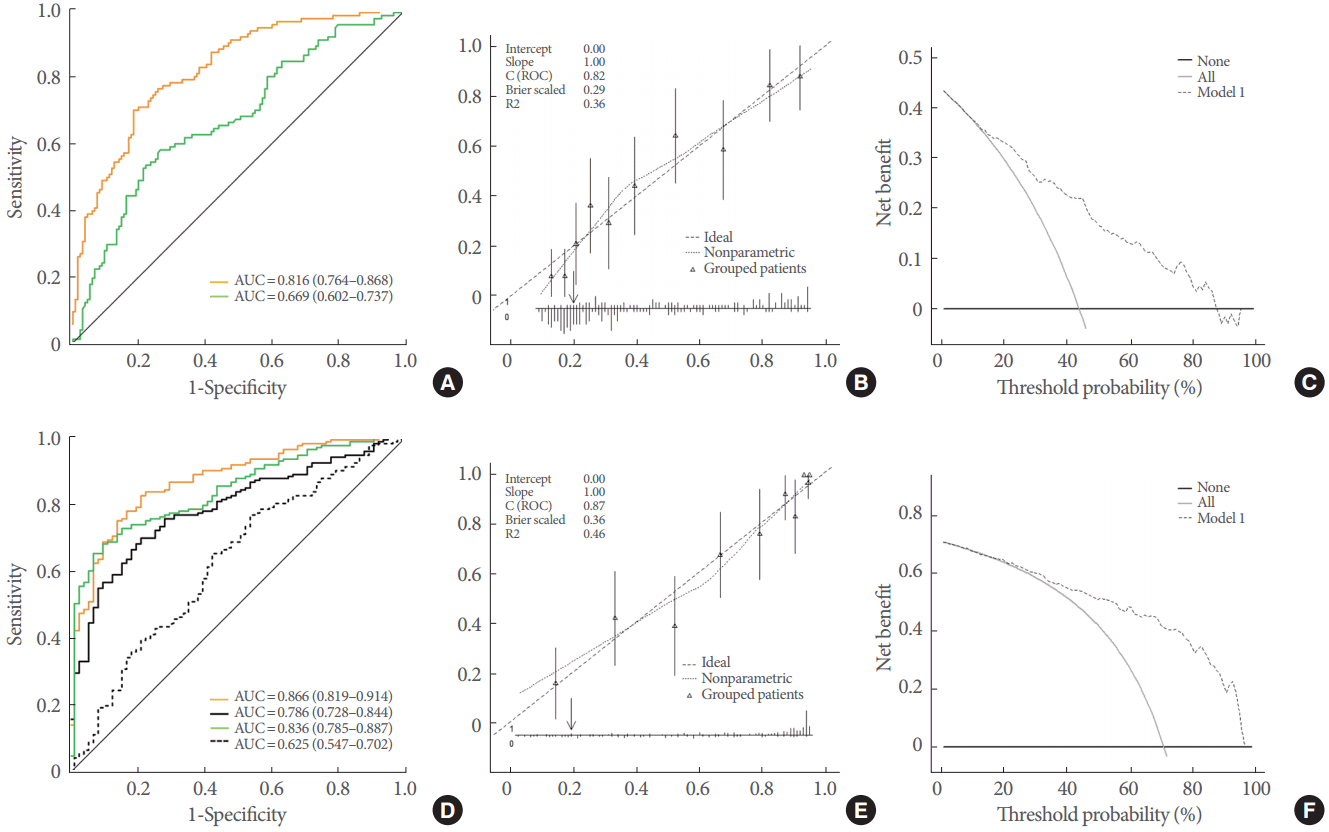
To evaluate the predictions based on the calculated probabilities, receiver operating characteristic curve analysis was applied to calculate the area under the curve (AUC) of the prediction, with AUCs of 0.70ŌĆō0.79 considered to indicate acceptable discrimination, 0.80ŌĆō0.89 to indicate excellent discrimination, and >0.90 to indicate outstanding discrimination [
9]. To assess calibration, calibration plots were generated to visualize the agreement between the predicted probability of both having BOO and requiring prostatic surgery, and the actual fact of both having BOO and undergoing prostatic surgery. Decision curve analysis was used to assess the clinical usefulness of each prediction model by quantifying the net benefits achieved by making decisions at each probability threshold [
10]. Sensitivity, specificity, the positive predictive value, and the negative predictive value were obtained and reported.
Two-sided P-values<0.05 were considered to indicate statistical significance. All statistical analyses were performed using R 3.3.1 (R Foundation for Statistical Computing, Vienna, Austria;
http://www.R-project.org), and the IBM SPSS Statistics ver. 23.0 (IBM Co., Armonk, NY, USA).
DISCUSSION
We conducted this study using a large database of urodynamic studies to develop formulas to predict BOO and the need for prostatic surgery. These formulas were calculated based on the urodynamic evidence of BOO. We developed 4 formulas, some of which can be used even when 1 or 2 parameters are not available. To the best of our knowledge, this is the first study of the development of a BOO-based decision-support prediction model for prostatic surgery in patients with LUTS/BPH.
Histologically, BPH corresponds to a proliferation of the stromal and epithelial cells in the prostate with aging. However, clinically, it is a heterogeneous condition composed of different aspects of LUTS, benign enlargement of the prostate, and functional BOO. It is well known that there are poor correlations among patients in terms of LUTS, BOO, and prostate volume [
11]. Although a patientŌĆÖs symptom severity or quality of life is important, these subjective factors are not absolutely reflective of objectively measurable parameters. For example, patients with detrusor underactivity without either prostate enlargement or BOO can have significant symptoms and bother. Therefore, a surgical decision based simply on the prostate volume or subjective LUTS might be inappropriate.
In patients with marginal clinical situations, urodynamically confirmed BOO can be used as an important guide in surgical decision-making. Urodynamic studies have been advocated as a useful predictor of the success of surgery, and this has been encouraged by international guidance [
12,
13]. Our meta-analysis likewise showed a significant association between urodynamically confirmed BOO and greater improvements in all treatment outcome parameters. Preoperative urodynamic studies may add insight into postoperative outcomes after the surgical treatment of BPH [
14]. BOO is significantly associated with both functional and morphological alterations of the urinary bladder. Animal experiments have demonstrated that chronic BOO can cause repeated bladder ischemia/reperfusion injuries, ultimately resulting in partial denervation of the bladder [
15,
16]. Such oxidative stress is responsible for bladder hyperactivity in moderate cases or detrusor underactivity, if it is prolonged [
17,
18]. Abundant clinical evidence has shown that BOO is significantly associated with functional changes in the urinary bladder. We analyzed the clinical and urodynamic characteristics of 429 elderly men with LUTS/BPH according to a urodynamic study. Patients with BOO had a significantly higher rate of involuntary detrusor contraction and poor compliance compared to the patients without BOO [
19]. Therefore, it is functional BOO that directly causes undesirable consequences, such as overactive bladder or underactive bladder. In addition, classic findings have also shown that urodynamically confirmed BOO is closely associated with morphological changes of the urinary bladder. A significant correlation was found between bladder trabeculation and the grade of BOO in old men with presumptive LUTS/BPH [
20,
21]. BOO confirmed through a urodynamic study was also closely related with radiologically demonstrated trabeculation [
21]. Therefore, bladder dysfunction secondary to BOO must be considered in the management of male patients with LUTS. The surgical treatment of patients who satisfy the absolute indications for surgery is often too late. This means that the conditions might be irreversible, in functional or morphological aspects, even after surgical relief.
In patients who do not meet the absolute indications for surgery, patient selection for surgery is often not easy. This decision may depend on the degree of bother of the LUTS, prostate volume, comorbidities, a patientŌĆÖs personal preferences, the financial status of the patient, the patientŌĆÖs willingness to accept potential surgery-associated complications, the experience of the surgeon, and the availability of a specific surgical armamentarium. In our institution, the decision to perform surgery in patients who did not meet the absolute indications was made more based on the presence of urodynamically confirmed BOO than on patient requests or prostate volume. We do not commonly recommend surgery to patients when they do not have significant BOO, even though the patient may have an enlarged prostate. PFS is considered as the gold standard for diagnosing BOO. Unfortunately, none of the noninvasive tests for diagnosing BOO in men with LUTS can currently be recommended as an alternative to a PFS [
22]. However, urodynamic studies are not recommended for routine clinical use due to their invasiveness, expense, time-consuming nature, and technical challenges [
23]. For this reason, we thought that a prediction model for BOO-based surgical decision-making without performing PFS would be very important. This study is an extension of our previous clinical studies involving BOO in patients with LUTS/BPH. We have performed several clinical studies using data that we have accumulated over the last 15 years. We sought to identify noninvasive parameters for predicting BOO using our clinical data, and found that Qmax played a significant role in predicting BOO in Korean men with LUTS [
19]. More recently, we also analyzed data from 1,381 patients with BPH using CBN analysis and found that TPV, Qmax, and PVR were independent predictive parameters of urodynamically confirmed BOO [
8].
One of the advantages of our urodynamic data is that the urodynamic studies were performed by the same investigators in a single center using the same practice protocol since 2002 when the International Continence Society standardization was published [
5]. In our institution, the prevalence of BOO among patients with LUTS/BPH is relatively high. We have a high number of patients who potentially need prostate surgery, as our hospital is a tertiary referral center with a heavy clinical volume. In this dataset, patients with an absolute indication for prostatic surgery were also included. This means that the clinical parameters of these patients were incorporated in these formulas. If patients did not agree to undergo surgery when the clinician recommended it, we still classified these patients as requiring prostatic surgery. The category of requiring surgery in this study solely reflects the decisions made by urologists.
A major limitation of our study is that only a few surgeons were involved in surgical decision-making in the BPH population. This might also have caused some bias in the surgical decisions, making these results not generalizable. However, the need for surgery determined by the surgeons is not essentially different from what would be expected based on textbook guidelines, since they followed the clinical guidelines published by the American Urological Association or the European Urological Association in their routine clinical practice [
2,
3]. Additionally, the need for surgery was not merely judged by the patientŌĆÖs request, but by considering the entire clinical situation of the patients, including more objective parameters such as urodynamically confirmed BOO. We believe that surgical decision-making in real clinical situations for LUTS/BPH patients whose conditions do not satisfy the absolute indications for surgery should be primarily based on the presence of functional obstruction. The purpose of surgery in patients with LUTS/BPH is to relieve conditions such as acute urinary retention, gross hematuria of prostatic origin, associated bladder stones, or hydronephrosis in patients who satisfy the absolute surgical indications for BPH. However, symptom improvement and overall patient satisfaction after prostate surgery are primary goals in patients who do not satisfy the absolute surgical indications. Based on our unpublished data, we found that the overall patient satisfaction after prostatectomy in patients with LUTS/BPH was very high (over 90%), with the current surgical decision- making process being based mainly on urodynamically confirmed BOO. We believe that our current process of selecting patients for surgery is reasonably justified.
We expect that this urodynamic BOO-based prediction model will be of great help in actual clinical practice. We believe that further validation in other ethnic populations is necessary, as this formula is based on an Asian population. Additionally, more research into subjective satisfaction and symptom improvement in patients who undergo prostatic surgery based on this nomogram is needed to justify our work.
In conclusion, we established nomograms for the prediction of BOO and the need for BOO-related surgery in patients with LUTS/BPH. Internal validation of the nomograms demonstrated that these nomograms predicted both BOO and requiring prostatic surgery very well. We hope that these BPH probability models will help clinicians to better select LUTS/BPH patients requiring surgical treatment.