 |
 |


- Search
| Int Neurourol J > Volume 27(2); 2023 > Article |
|
ABSTRACT
Purpose
To compare improvement of lower urinary tract symptoms (LUTS) due to benign prostatic hyperplasia in diabetic versus nondiabetic patients after transurethral resection of the prostate (TURP) or holmium laser enucleation of the prostate (HoLEP).
Methods
The medical records of 437 patients who underwent TURP or HoLEP at a tertiary referral center from January 2006 to January 2022 were retrospectively analyzed. Among them, 71 patients had type 2 diabetes. Patients in the diabetic mellitus (DM) and non-DM groups were matched 1:1 according to age, baseline International Prostate Symptom Score (IPSS), and ultrasound measured prostate volume. Changes in LUTS were assessed at 3 months after surgery using IPSS and evaluated by categorizing patients according to prostatic urethral angulation (PUA; <50┬░ vs. Ōēź50┬░). Medication-free survival after surgery was also investigated.
Results
No significant differences were noted between the DM and non-DM groups in baseline characteristics except for comorbidities (i.e., hypertension, cerebrovascular disease, and ischemic heart disease, P=0.021, P=0.002, and P=0.017, respectively) and postvoid residual urine volume (115┬▒98 mL vs. 76┬▒105 mL, P=0.028). Non-DM patients showed significant symptomatic improvement regardless of PUA, while DM patients demonstrated improvement in obstructive symptoms only in those with large PUA (Ōēź51┬░). Among patients with small PUA, DM patients had worse medication-free survival after surgery compared to controls (P=0.044) and DM was an independent predictor of medication reuse (hazard ratio, 1.422; 95% confidence interval, 1.285ŌĆō2.373; P=0.038).
Benign prostatic hyperplasia (BPH) is a common condition in aging men. An estimated 50%ŌĆō70% may have histologic evidence of this disease by the age of 50ŌĆō80 years and approximately half of them manifest significant lower urinary tract symptoms (LUTS) [1]. The prevalence of BPH is expected to increase rapidly in the United States [2] and other parts of the developed world, including western Europe and northeast Asia, due to aging populations, making the management of BPH a great public health concern [3]. Meanwhile, type 2 diabetes mellitus (DM) is another great health challenge that is just as common as BPH in the elderly population and its negative impact on bladder function is well known [4]. Because both BPH and diabetes are prevalent in the aging male, the management of this patient group with concomitant BPH and diabetes has become an important issue in recent years.
The effect of diabetes on LUTS is well understood. Diabetic autonomic neuropathy exacerbates urinary bladder contractions, which cause worsening of urinary symptoms, such as urine hesitancy, urgency, and recurrent urinary infections [5]. Specifically, bladder dysfunction related to diabetes is associated with anatomical/functional alteration of urothelium, the detrusor muscle, and innervation of the urinary bladder [6]. Accordingly, a previous study showed that, after adjusting for age, men with diabetes were 1.42 times more likely to report moderate to severe LUTS and showed a significantly lower maximum urinary flow rate than nondiabetic men [7,8]. Therefore, it is quite possible that diabetes may be an etiology of BPH or at least it may be related to the process and complications of BPH [9].
In the management of BPH, medical therapy such as treatment with alpha blockers, is the most common first-line treatment and it has acceptable efficacy, safety, and outstanding tolerance [10]. However, patients with a poor response to medical therapy may require surgical intervention, such as transurethral resection of the prostate (TURP) or holmium laser enucleation of the prostate (HoLEP) [11,12]. Although the association between BPH/LUTS and diabetes has been vigorously discussed in the literature, very few studies have focused on the effect of diabetes on outcomes after surgery for BPH. Therefore, in this study, we compared the treatment outcomes of diabetic patients undergoing surgical intervention for treatment of BPH with treatment outcomes of nondiabetic counterparts. Furthermore, as it is well known that prostatic urethral angulation (PUA) would affect surgical outcome which was also shown in our previous study [13], we investigated the influence of PUA on symptom improvement after surgery in patients with and without diabetes.
The medical records of 590 patients who underwent TURP or HoLEP from January 2006 to January 2022 were retrospectively reviewed. Patients with conditions that could affect voiding function or symptoms such as prostate or bladder cancer, uncontrolled DM (fasting plasma glucose >250 mg/dL, consecutive random plasma glucose >300 mg/dL, or HbA1c >10%), neurologic diseases such as ParkinsonŌĆÖs disease, current urinary tract infection, bladder stones, urethral stricture, history of lower urinary tract surgery or pelvic irradiation, or incomplete medical records were excluded. A total of 437 patients were finally included in the analysis. The baseline parameters of the patients prior to surgery were recorded and included in the analysis, which were age, serum prostate-specific antigen (PSA) level, comorbidities, International Prostate Symptom Score (IPSS), maximal uroflow rate (Qmax), postvoid residual urine volume measured by diagnostic ultrasound, prostate volume measured by transrectal ultrasonography (TRUS), and urologic medication history (╬▒-blocker, antimuscarinic, and bethanechol chloride).
The severity of LUTS was assessed with IPSS, and the total score (IPSS-t) and separated scores, such as voiding symptom (IPSS-vs), storage symptom (IPSS-ss), and quality of life (IPSSQoL) subscores, were recored. We applied methodologies previously described to assess other parameters associated with voiding symptoms or functions, including uroflowmetry, prostate volume, and PUA [13]. PUA was specifically determined by measuring the angle formed by 2 rays of the proximal and distal prostatic urethra on midsagittal plane, obtained using TRUS. A bipolar TURP was performed conventionally with saline irrigation in all cases using a bipolar current generator and wire loop (Gyrus Medical GMBH, Tuttligen, Germany) with a 24F resectoscope (Karl Storz Endoscope, Tuttlingen, Germany). The cutting and coagulation powers were within 160 W and 80 W, respectively. A 20F 3-way silicone urethral catheter was placed in all patients postoperatively to maintain saline irrigation until hematuria resolved. A 26F continuous flow resectoscope and a laser-fiber stabilizing bridge, a 100 W holmium laser (VersaPulse, Lumenis Ltd., Yokneam, Israel) with a 550-╬╝m fiber (SlimLine, Lumenis Ltd.) was used for HoLEP. Power settings were 80 W for cutting and 40 W for coagulation. A 26F nephroscope and a tissue morcellator (VersaCut, Lumenis Co.) were used to remove tissue after enucleation.
The primary goal of this study was to assess effect of DM on surgical outcome which was assessed by IPSS and therefore patients eligible for analysis were classified into 2 groups, i.e., non-DM and DM groups. For comparison, propensity scores were calculated for each subject using logistic regression based on the patientsŌĆÖ age, preoperative IPSS, and prostate volume measured by TRUS to adjust for imbalances in preoperative characteristics that may affect surgical outcomes. Patients in the 2 groups were matched with a 1:1 ratio based on propensity scores. The distribution of the demographic characteristics and preoperative variables between the groups were compared with an independent t-test for continuous variables and a chi-square test for categorical variables. Impact of PUA on the changes in LUTS before and after surgery were evaluated by subcategorizing patients according to PUA (<51┬░ vs. Ōēź51┬░) followed by IPSS assessment, which was measured before and at 3 months after surgery. Finally, the medication-free survival rate defined as discontinuation of BPH-related medication including ╬▒-blocker, 5╬▒ reductase inhibitors, and/or antimuscarinics after surgery was estimated with Kaplan-Meier analysis and the non-DM and DM groups were compared using a log-rank test. Univariate and multivariate analyses were conducted using Cox proportional hazard models to identify independent predictive variables of medication-free survival. All tests were 2-sided, and P-values <0.05 were considered statistically significant. IBM SPSS Statistics ver. 22.0 (IBM Co., Armonk, NY, USA) was used to conduct the statistical analyses.
There were 366 (83.8%) and 71 patients (16.2%) in the non-DM and DM groups, respectively, who received either TURP (279, 76.2%) or HoLEP (158, 43.2%). Patients in both groups were matched 1:1 according to age, baseline IPSS, and the ultrasound prostate volume; 71 patients were in each group. Baseline characteristics that showed significant differences between the groups before matching including the serum PSA level, prostate volume, and urologic medications such as ╬▒-blockers, demonstrated no significant differences after matching (Table 1). The proportion of patients with comorbidities associated with the circulatory system (e.g., hypertension, cerebrovascular disease, and ischemic heart disease) and postvoid residual urine volume (76┬▒105 vs. 115┬▒98, P=0.028, non-DM vs. DM group, respectively) remained, which showed significant differences after matching. There were no significant differences in baseline IPSS both before and after matching.
The analysis on the total patients showed that all domains in IPSS including IPSS-t, IPSS-vs, and IPSS-ss demonstrated significant improvements (-8.2, -5.8, and -3.1, respectively, all P<0.05) in non-DM patients. In DM patients, IPSS-t and IPSS-vs showed significant improvements (-2.7 and -2.9, respectively, all P<0.05) (Fig. 1A). Similarly in patients with large PUA (Ōēź51┬░), IPSS-t, IPSS-vs, and IPSS-ss all showed significant improvements as a whole (-11.2, -5.4, and -3.2, respectively, all P<0.05) in non-DM patients. In addition, IPSS-t and IPSS-vs improved significantly (-4.6 and -5.5, respectively, all P<0.05) in DM patients (Fig. 1B). IPSS-QoL did show improvement in non-DM patients, although it was not statistically significant. However, in patients with small PUA (<51┬░), IPSS-t, IPSS-vs, and IPSS-ss all demonstrated significant improvements at 3 months after surgery (-6.8, -5.1, and -2.9, respectively, all P<0.05) in non-DM patients, while there were no significant improvements in all 3 domains of IPSS in DM patients (Fig. 1C).
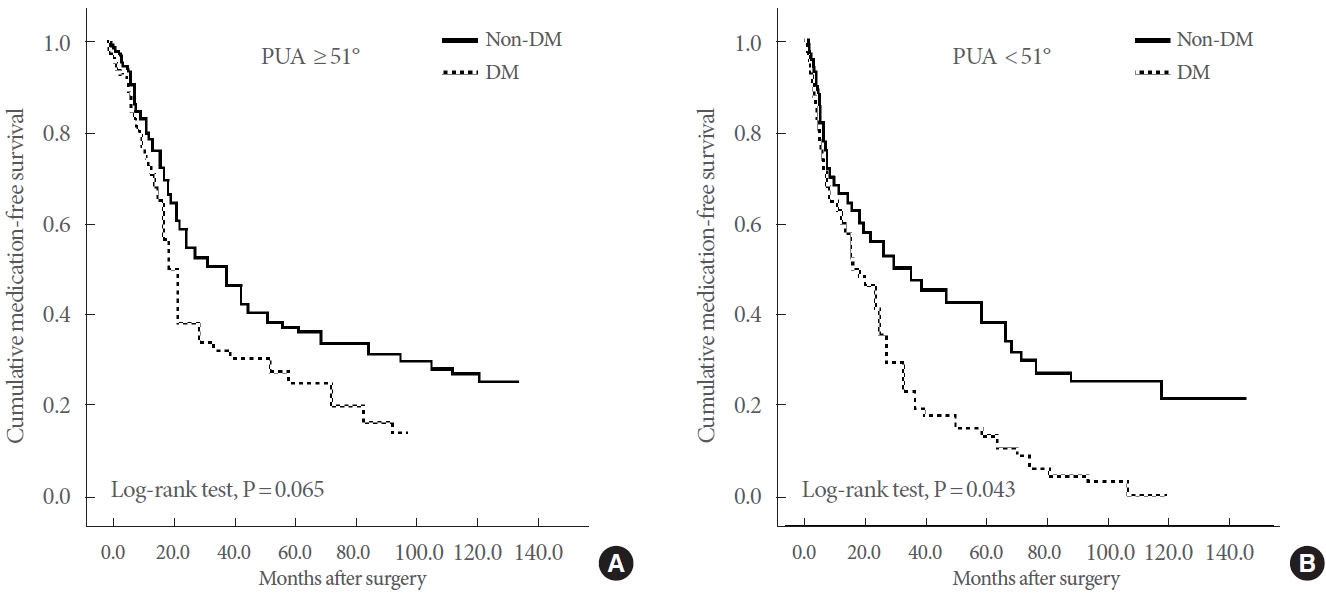
Since the optimal outcome of BPH surgery is considered to be cessation of urologic drugs after surgery, the medication-free survival rates were compared between the non-DM and DM patient groups during the mean follow-up time of 5.7 and 5.1 years, respectively. The results indicated that, although there was a tendency towards worse medication-free survival of DM patients compared to those of non-DM patients, it was not statistically significant in patients with large PUA (Ōēź51┬░) (log-rank test, P=0.065) (Fig. 2A). Contrarily, an analysis of the patients with small PUA (<51┬░) demonstrated a significant worse medication-free survival in DM patients when compared with their counterparts (log-rank test, P=0.044) (Fig. 2B). A multivariate analysis of the patients with small PUA showed that DM was an independent predictor of medication reuse (hazard ratio, 1.422; 95% confidence interval, 1.285ŌĆō2.373; P=0.038) (Table 2), along with age, preoperative IPSS, and prostate volume (P=0.012, P=0.011, and P<0.001, respectively) (Table 2). Unlike DM, other comorbidities including cerebrovascular disease failed to predict medication reuse in such patient group.
Researchers have suggested a possible link between diabetes and BPH/LUTS for the last 2 decades and multiple disparate and interrelated mechanisms were considered [14]. Accordingly, diabetes-induced insulin resistance and consequent hyperinsulinemia may increase sympathetic nerve activity and eventually lead to increases in the prostate smooth muscle tone and bladder outlet obstruction [15]. Furthermore, both hyperinsulinemia with dysregulation of the insulin-like growth factor and the proinflammatory effect of hyperglycemia associated with diabetes can also induce overgrowth of prostatic tissue, therefore aggravating BPH/LUTS [16,17]. Indeed, several studies have demonstrated an association of BPH/LUTS with diabetesrelated hyperglycemia and insulin resistance [7,8,18].
BPH surgery, such as TURP or HoLEP, may be considered if LUTS do not improve despite adequate medication or if the patients are not willing to receive continuous, regular medication. However, previous studies have indicated that detrusor underactivity before surgery, which can often be found in DM patients, are associated with inferior surgical results [19]. Therefore, it is reasonable to anticipate that DM patientsŌĆÖ LUTS would be relieved slower, especially storage symptoms after surgery compared to their non-DM counterparts. Nevertheless, very few studies have focused on the impact of diabetes on surgical outcomes in BPH patients. One Taiwanese study using a large cohort database of 4,887 patients who underwent TURP showed that patients with DM had higher rates of urinary retention requiring catheterization within one month after surgery and required higher rates of continuing medication at 3 months postoperatively [20]. Although these results have significance suggesting a correlation between diabetes and surgical outcomes, it seems insufficient for practical use because there is a lack of information on the association between DM and surgical outcomes under specific circumstances, including prostate volume and prostatic anatomy, such as PUA. Also, previous studies did not provide information on specific changes in LUTS (e.g., voiding and storage symptoms) after surgery in patients with or without diabetes.
In our study, surgical outcomes of BPH surgery (TURP or HoLEP) were specifically focused on patients with DM compared to non-DM counterparts and it was expressed with changes in IPSS. In addition, other factors that could possibly influence the results were investigated further. Our results revealed some differences in the characteristics between the patient groups that underwent BPH surgery and that DM patients have a higher incidence of diseases associated with the circulatory system (hypertension, cerebrovascular disease, and ischemic heart disease) and larger prostate volume and PUA. There was no significant difference regarding age at surgery and overall baseline IPSS between the 2 groups. In order to precisely analyze the effect of DM on surgical outcomes, we conducted propensity score matching for factors that could affect urinary tract symptoms such as age, preoperative IPSS, and prostate volume to control possible confounding effects.
Analysis of IPSS changes after surgery revealed that patients with small PUA and DM did not benefit significantly from the surgery whereas DM patients with large PUA demonstrated significant improvements regarding IPSS-t and IPSS-vs, although IPSS-ss did not show improvements. These findings are consistent with previous studies that have considered the association of diabetes with the idea that long-standing hyperglycemia might lead to worsening of storage symptoms through sympathetic nervous system stimulation, and diabetes patients can also be characterized by impaired bladder sensations and decreased contractility of the bladder [21]. In the same context, our results indicated that, although DM patients with large PUA can achieve overall symptom improvements unlike those with small PUA, storage symptoms would remain unimproved after surgery. In addition, our current findings not only suggest a link between DM and surgical outcomes in BPH patients, they also imply an effect of PUA on surgical outcomes of patients who have both DM and BPH/LUTS. Kaplan-Meier plots further confirmed implications of PUA in that DM patients showed poorer medication-free survival compared to non-DM patients only among those with small PUA. These findings imply that DM may not influence the surgical outcome in patients with large PUA, probably because these patients might significantly benefit from BPH surgery regardless of their DM status. However, on the other hands, it is assumed that the negative effect of DM on the surgical outcome may be relatively greater in patients with small PUA compared to those with large PUA.
An association between PUA and LUTS has already been reported in several previous studies that showed a large PUA indicated aggravated bladder outlet obstruction. According to our previous results, better outcomes after surgery were associated with greater PUA in patients with a relatively small prostate (<50 mL), suggesting that the importance PUA on the outcome may be even greater in patients with a small prostate [13]. Referring to those previous studies and our current results regarding diabetes and PUA, it is expected that both PUA and diabetic status can affect the surgical outcome, and therefore considering PUA in the decision-making process in diabetic patients may lead to optimal results.
This study has some limitations, which include the retrospective nature of the analysis and lack of personal information, such as alcohol/caffeine consumption, cigarette use, and exercise, that may influence urinary tract symptoms. More importantly, HgA1c, the duration of DM, and objective parameters such as Qmax and postvoid residual urine volume after surgery was not taken into consideration due to incompleteness of the medical records. Objective parameters such was not included in the analysis. In addition, by not including the urodynamic study results, it was impossible to completely rule out the possibility that neurogenic bladder patients were included in the study. However, we tried to avoid this by checking the residual urine result of uroflowmetry, performing matching for IPSS, and excluding patients with uncontrolled DM. Nevertheless, in this study, the effect of diabetes on the surgical outcome was quantitatively analyzed using IPSS and considering the volume and morphology of the prostate, which can also affect the surgical outcome. Therefore, it is expected that novel and efficient guidelines can be developed for decision making in the treatment of BPH on the basis of the current study. In conclusion, patients with diabetes may present with unsatisfactory symptom improvement. However, among patients with large PUA, even patients with diabetes may expect improvement in their symptoms after surgery. In addition, patients with diabetes and small PUA are more likely to continue BPH-related medications after surgery than their non-DM counterparts.
NOTES
Grant/Fund Support
This research was supported by Hallym University Research Fund 2020 (HURF-2021-41).
Research Ethics
This study was undertaken with the approval by the institutional review board of Hallym University Hospital (approval number, 2022-02-015).
AUTHOR CONTRIBUTION STATEMENT
ŌĆó Conceptualization: JKJ, MS
ŌĆó Data curation: JKJ, HK, WJB, CYO, JSC, MS
ŌĆó Formal analysis: JKJ, MS
ŌĆó Funding acquisition: MS
ŌĆó Methodology: JKJ, MS
ŌĆó Project administration: HK, WJB, CYO, JSC, MS
ŌĆó Visualization: HK, JSC, MS
ŌĆó Writing - original draft: JKJ
ŌĆó Writing - review & editing: MS
REFERENCES
1. Guess HA, Arrighi HM, Metter EJ, Fozard JL. Cumulative prevalence of prostatism matches the autopsy prevalence of benign prostatic hyperplasia. Prostate 1990;17:241-6. PMID: 1700403


2. Litman HJ, McKinlay JB. The future magnitude of urological symptoms in the USA: projections using the Boston Area Community Health survey. BJU Int 2007;100:820-5. PMID: 17550412


3. Jo JK, Shinn SH, Kim KS, Moon HS. Changes in prevalence and treatment pattern of benign prostatic hyperplasia in Korea. Int Neurourol J 2021;25:347-54. 


4. Sarma AV, Kellogg Parsons J. Diabetes and benign prostatic hyperplasia: emerging clinical connections. Curr Urol ReP 2009;10:267-75. 

5. Pourghasem M, Shafi H, Babazadeh Z. Histological changes of kidney in diabetic nephropathy. Caspian J Intern Med 2015;6:120-7. PMID: 26644877


6. Sasaki K, Yoshimura N, Chancellor MB. Implications of diabetes mellitus in urology. Urol Clin North Am 2003;30:1-12. PMID: 12580554


7. Sarma AV, Burke JP, Jacobson DJ, McGree ME, St Sauver J, Girman CJ, et al. Associations between diabetes and clinical markers of benign prostatic hyperplasia among community-dwelling black and white men. Diabetes Care 2008;31:476-82. PMID: 18071006



8. Michel MC, Mehlburger L, Schumacher H, Bressel HU, Goepel M. Effect of diabetes on lower urinary tract symptoms in patients with benign prostatic hyperplasia. J Urol 2000;163:1725-9. PMID: 10799169


9. Stamatiou K, Lardas M, Kostakos E, Koutsonasios V, Michail E. The impact of diabetes type 2 in the pathogenesis of benign prostatic hyperplasia: a review. Adv Urol 2009;2009:818965. PMID: 19902013




10. Lin KH, Lin YW, Wen YC, Lee LM. Efficacy and safety of orally disintegrating tamsulosin tablets in Taiwanese patients with benign prostatic hyperplasia. Aging Male 2012;15:246-2. PMID: 23067262


11. Kim EH, Larson JA, Andriole GL. Management of benign prostatic hyperplasia. Annu Rev Med 2016;67:137-51. PMID: 26331999


12. Christidis D, McGrath S, Perera M, Manning T, Bolton D, Lawrentschuk N. Minimally invasive surgical therapies for benign prostatic hypertrophy: the rise in minimally invasive surgical therapies. Prostate Int 2017;5:41-6. PMID: 28593165



13. Shim M, Bang WJ, Oh CY, Lee YS, Cho JS. Correlation between prostatic urethral angulation and symptomatic improvement after surgery in patients with lower urinary tract symptoms according to prostate size. World J Urol 2020;38:1997-2003. PMID: 31646381



14. Hammarsten J, Peeker R. Urological aspects of the metabolic syndrome. Nat Rev Urol 2011;8:483-94. PMID: 21811224



15. McVary KT, Rademaker A, Lloyd GL, Gann P. Autonomic nervous system overactivity in men with lower urinary tract symptoms secondary to benign prostatic hyperplasia. J Urol 2005;174:1327-433. PMID: 16145413


16. Sampson N, Zenzmaier C, Heitz M, Hermann M, Plas E, Schafer G, et al. Stromal insulin-like growth factor binding protein 3 (IGFBP3) is elevated in the diseased human prostate and promotes ex vivo fibroblast-to-myofibroblast differentiation. Endocrinology 2013;154:2586-99. PMID: 23720424


17. Jangir RN, Jain GC. Diabetes mellitus induced impairment of male reproductive functions: a review. Curr Diabetes Rev 2014;10:147-57. PMID: 24919656


18. Parsons JK, Carter HB, Partin AW, Windhan BG, Metter EJ, Ferrucci L, et al. Metabolic factors associated with benign prostatic hyperplasia. J Clin Endocrinol Metab 2006;91:2562-8. PMID: 16608892



19. Zhu Y, Zhao YR, Zhong P, Qiao BM, Yang ZQ, Niu YJ. Detrusor underactivity influences the efficacy of TURP in patients with BPO. Int Urol Nephrol 2021;53:835-41. PMID: 33386583



20. Lin YH, Hou CP, Chen TH, Juang HH, Chang PL, Yang PS, et al. Is diabetes mellitus associated with clinical outcomes in aging males treated with transurethral resection of prostate for bladder outlet obstruction: implications from Taiwan Nationwide Populationbased cohort study. Clin Interv Aging 2017;12:535-41. PMID: 28356725




21. Choi H, Bae JH, Oh CY, Jeong SJ, Ko WJ, Choi JB, et al. Clinical efficacy of solifenacin in the management of diabetes mellitus-associated versus idiopathic overactive bladder symptoms: a multicenter prospective study. Int Neurourol J 2018;22:51-7. PMID: 29609421




Fig.┬Ā1.
Mean (┬▒standard error) values of the International Prostate Symptom Scores (IPSS) from baseline to 3 months after surgery (non-DM vs. DM patients), stratified by prostatic urethral angulation (PUA). Total (A), PUA Ōēź51┬░ (B), and PUA <51┬░ (C). DM, diabetes mellitus.

Fig.┬Ā2.
Medication-free survival in patients with PUA Ōēź51┬░ (A) and PUA <51┬░ (B), as estimated using the Kaplan-Meier method. PUA, prostatic urethral angulation; DM, diabetes mellitus.
Table┬Ā1.
Patient characteristics in the non-DM and DM groups before and after matching
| Characteristic |
Non-DM |
DM (n=71) |
P-value |
|||
|---|---|---|---|---|---|---|
| Unmatched (n=366) | Matched (n=71) | Unmatched | Matched | |||
| Age (yr)a) | 67.6 ┬▒ 10.8 | 67.4 ┬▒ 11.5 | 67.2 ┬▒ 10.6 | 0.875 | 0.987 | |
| Serum PSA level (ng/mL) | 1.6 ┬▒ 1.4 | 2.5 ┬▒ 1.6 | 2.8 ┬▒ 1.8 | 0.015* | 0.096 | |
| Fasting blood glucose level (mg/dL) | 87.6 ┬▒ 10.2 | 92.3 ┬▒ 19.1 | 109 ┬▒ 38.1 | 0.008* | 0.034* | |
| Comorbidity | ||||||
| Hypertension | 146 (39.9) | 30 (42.3) | 48 (67.6) | 0.018* | 0.021* | |
| Cerebrovascular disease | 32 (8.7) | 13 (18.3) | 14 (19.7) | < 0.001* | 0.322 | |
| Chronic obstructive pulmonary disease | 40 (10.9) | 11 (15.5) | 10 (14.1) | 0.348 | 0.594 | |
| Ischemic heart disease | 46 (12.6) | 12 (16.9) | 13 (18.3) | 0.036* | 0.217 | |
| IPSS, baselinea) | ||||||
| Total | 16.8 ┬▒ 8.7 | 17.9 ┬▒ 5.6 | 18.3 ┬▒ 6.3 | 0.098 | 0.542 | |
| Voiding symptoms | 9.5 ┬▒ 6.7 | 10.3 ┬▒ 5.9 | 10.5 ┬▒ 5.2 | 0.131 | 0.976 | |
| Storage symptoms | 4.3 ┬▒ 3.7 | 5.2 ┬▒ 4.1 | 5.3 ┬▒ 4.6 | 0.063 | 0.873 | |
| Quality of life | 3.5 ┬▒ 2.2 | 3.8 ┬▒ 3.6 | 3.9 ┬▒ 3.1 | 0.782 | 0.985 | |
| Uroflowmetry, baseline | ||||||
| Maximum flow rate (mL/sec) | 14.8 ┬▒ 4.2 | 13.2 ┬▒ 6.3 | 12.8 ┬▒ 5.9 | 0.097 | 0.195 | |
| Voided volume (mL) | 225 ┬▒ 124 | 228 ┬▒ 158 | 231 ┬▒ 167 | 0.798 | 0.875 | |
| Postvoid residual urine volume (mL) | 68 ┬▒ 53 | 76 ┬▒ 105 | 115 ┬▒ 98 | 0.007* | 0.028* | |
| Transrectal ultrasonography | ||||||
| TPV (mL)a) | 52.5 ┬▒ 35.4 | 63.8 ┬▒ 31.5 | 65.4 ┬▒ 33.8 | 0.008* | 0.154 | |
| TZV (mL) | 23.2 ┬▒ 15.7 | 31.3 ┬▒ 17.4 | 32.9 ┬▒ 21.1 | 0.013* | 0.325 | |
| PUA (┬░) | 45.6 ┬▒ 6.8 | 52.7 ┬▒ 7.8 | 53.2 ┬▒ 7.1 | 0.045* | 0.584 | |
| Medication within 3 months | ||||||
| ╬▒-blockers | 285 (77.9) | 57 (80.3) | 58 (81.7) | 0.039* | 0.575 | |
| Antimuscarinics | 47 (12.8) | 10 (14.1) | 13 (18.3) | 0.072 | 0.267 | |
| Bethanechol | 41 (11.2) | 10 (14.1) | 11 (15.5) | 0.028* | 0.796 | |
| Surgery | 0.486 | 0.752 | ||||
| TURP | 236 (64.5) | 45 (63.4) | 43 (60.6) | |||
| HoLEP | 130 (35.5) | 26 (36.6) | 28 (39.4) | |||
Values are presented as mean┬▒standard deviation or number (%).
DM, diabetes mellitus; PSA, prostate-specific antigen; IPSS, international prostate symptom score; TPV, total prostate volume; TZV, transitional zone volume; PUA, prostatic urethral angle; TURP, transurethral resection of prostate; HoLEP, holmium laser enucleation of prostate.
Table┬Ā2.
Cox proportional hazard model for predictors of medication reuse in patients with prostatic urethral angle <51┬░
| Variable |
Univariate |
Multivariate |
|||
|---|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | ||
| Age | 1.092 (1.003ŌĆō1.121) | < 0.001* | 1.121 (1.008ŌĆō1.228) | 0.010* | |
| Preoperative serum PSA level | 1.952 (0.358ŌĆō3.255) | 0.195 | 1.842 (0.631ŌĆō2.757) | 0.261 | |
| Comorbidity | |||||
| Hypertension | 1.827 (0.524ŌĆō3.156) | 0.652 | 1.751 (0.423ŌĆō3.237) | 0.753 | |
| Cerebrovascular disease | 2.286 (0.908ŌĆō3.523) | 0.359 | 1.871 (0.909ŌĆō3.691) | 0.431 | |
| Chronic obstructive pulmonary disease | 1.268 (0.857ŌĆō3.084) | 0.291 | 1.321 (0.798ŌĆō3.992) | 0.311 | |
| Ischemic heart disease | 2.521 (0.528ŌĆō5.131) | 0.314 | 3.131 (0.621ŌĆō5.218) | 0.378 | |
| Diabetes mellitus | 1.538 (1.326ŌĆō2.437) | 0.031* | 1.426 (1.289ŌĆō2.361) | 0.035* | |
| Preoperative IPSS (19 or less vs. 20 or higher) | 0.652 (0.402ŌĆō0.983) | 0.009* | 0.774 (0.502ŌĆō0.991) | 0.012* | |
| Maximum flow rate in uroflowmetry (13 mL/sec or less vs. higher than 13 mL/sec) | 3.082 (1.428ŌĆō3.982) | 0.025 | 3.009 (1.000ŌĆō3.282) | 0.053 | |
| Postvoid residual urine volume | 1.358 (0.982ŌĆō1.608) | 0.824 | 1.321 (0.895ŌĆō1.619) | 0.915 | |
| Prostate volume (less than 55 mL vs. 55 mL or higher) | 4.872 (3.721ŌĆō6.623) | < 0.001* | 4.491 (3.632ŌĆō6.781) | < 0.001* | |





