Management of Urinary Incontinence With Underactive Bladder: A Review
Article information
Abstract
Urinary incontinence is caused by storage function failure, while underactive bladder (UAB) is caused by a decline in detrusor contractility and voiding dysfunction. As the treatment mechanisms for incontinence and UAB are contrary to each other, it is difficult to treat both incontinence and UAB, and the patient’s quality of life can be further degraded. Conventional midurethral sling (MUS), such as transobturator tape or retropubic MUS, introduces a risk of postoperative voiding dysfunction in stress urinary incontinence with UAB. However, there have been several reports about the efficacy and safety of conventional MUS. Adjustable sling procedures, such as transobturator adjustable tape or the Remeex system, have better outcomes than conventional MUS because they control tension both during and after surgery. When voiding dysfunction occurs after incontinence treatment with UAB, voiding symptoms can be improved by various therapeutic modalities. Clean intermittent catheterization is recommended for patients with significant increased postvoid residual volumes or urinary retention. Although pharmacotherapy such as with alpha-blockers or parasympathomimetics can be considered for UAB, there is insufficient evidence of their effect on incontinence with UAB. Future therapies, such as stem cell therapy or gene therapy, may be used to treat incontinence with UAB. The possibility of management urgency urinary incontinence that related to detrusor hyperactivity with impaired contractility using sacral neuromodulation has been suggested. Further research is needed to establish evidence for the efficacy and safety of treatments for incontinence with UAB and improve patient quality of life.
INTRODUCTION
Urinary incontinence has the largest negative effect on patient quality of life among lower urinary tract symptoms (LUTS) [1]. Incontinence can coexist with other lower urinary tract dysfunction, such as overactive bladder (OAB) or underactive bladder (UAB). While OAB can be controlled to a certain extent through pharmacotherapy such as with antimuscarinics, there is a lack of definitive treatment for UAB. In addition, the UAB treatment mechanism is contrary to that for incontinence, which makes it difficult to treat incontinence with UAB.
Although an expert group defined UAB as a symptom complex related to detrusor underactivity (DU) that is usually characterized by prolonged urination time with or without a sensation of incomplete bladder emptying, usually with hesitancy, reduced bladder filling sensation, slow stream, straining to void, enuresis, and/or stress incontinence [2], its definition has not yet been standardized. Because of the variability in definition, the reported prevalence of incontinence with UAB also varies. A prevalence study of DU and stress urinary incontinence (SUI) among elderly patients with LUTS in Korea showed that more than 40% of women with DU had accompanying SUI [3]. Ong and Kuo [4] performed a preoperative video urodynamic study (UDS) of women with SUI and reported that 19.4% of the enrolled patients had detrusor overactivity (DO) and 8.4% had DU.
Conventional treatment for incontinence may result in different outcomes for patients with UAB, and other treatments or a whole new method may be required. This review focuses on treatment options and their outcomes for incontinence with UAB.
PATHOPHYSIOLOGY
Although the pathophysiology of UAB is not yet clearly defined, the main mechanism is decline in the detrusor contractile function due to myogenic failure, efferent nerve dysfunction, afferent nerve dysfunction, and failure of the central nervous system to coordinate voiding function [5]. Urinary incontinence is caused by a failure of the storage function of the lower urinary tract that is related to abnormal detrusor activity, such as DO or inadequate bladder outlet pressure. Urethral hypermobility and intrinsic sphincter deficiency have been suggested as the major mechanisms of incontinence due to abnormal bladder outlet function [6].
The urodynamic findings of UAB include DU, an acontractile detrusor, and detrusor hyperactivity with impaired contractility (DHIC). Although DHIC is associated with urgency urinary incontinence (UUI), it does not account for much of the UAB population [7]. SUI, a type of urethral sphincter dysfunction, is more likely to be associated with UAB involving incontinence.
Incontinence treatment requires decreased detrusor pressure or increased bladder outlet pressure, but UAB treatment requires increased detrusor pressure or decreased bladder outlet pressure. Therefore, UAB treatment and incontinence treatment may involve contradictory mechanisms, complicating their combined treatment. Treatments for SUI inhibit urinary leakage by increasing bladder outlet resistance or restoring the natural shape and configuration of the urethral support. One complication, regardless of the type of anti-incontinence surgery, is iatrogenic obstruction, which has an incidence of 2.5%– 24% [8]. In patients with SUI and UAB, there is a concern for developing obstructive voiding problems that will require sling revision or removal. There are no clear treatment guidelines for SUI with UAB. It is important to maintain proper lower urinary tract function by controlling the severity or degree of incontinence and UAB. This should be supported by accurate UDS findings prior to the treatment of incontinence with UAB.
MANAGEMENT
Conservative Treatment
Pelvic floor muscle training (PFMT) for patients with urinary incontinence can reduce urine leakage episodes and improve quality of life without increasing negative side effects [9]. While PFMT is the first-line treatment for urinary incontinence, it is not efficacious in moderate or severe incontinence compared to surgical treatment such as a midurethral sling (MUS) [10]. Some studies have shown the potential of PFMT with biofeedback for UAB [11,12]. Conservative treatment, such as PFMT alone, may not be effective in patients with UAB incontinence. However, the benefits of conservative treatment may be useful in combination with other treatments, although the evidence remains insufficient.
Conventional Midurethral Sling
Conventional MUS, which controls SUI by the placement of a mesh at the midurethra through either the obturator or retropubic space, has a long-term subjective cure rate of 43% to 92% [13]. However, the efficacy and safety of conventional MUS in patients with SUI coexisting with UAB may be different.
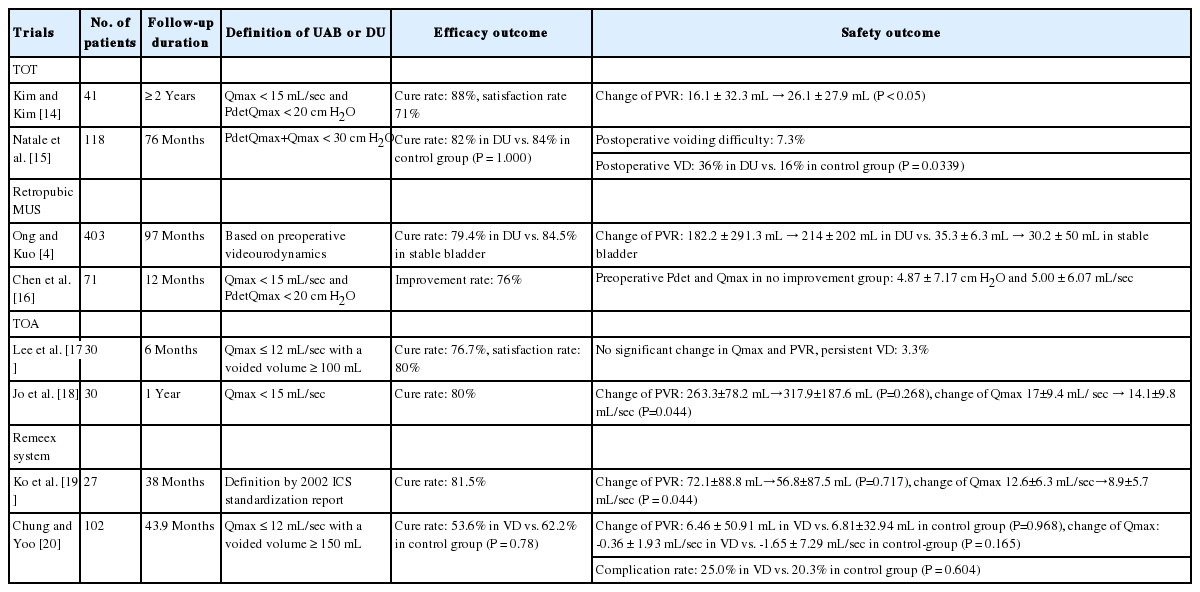
Kim and Kim [14] showed that transobturator tape (TOT) could be installed safely for patients with SUI and DU, with 88% success and 7% dissatisfaction rates over more than 2 years of follow-up. In that study, DU was defined by a maximal flow rate (Qmax) of<15 mL/sec and detrusor pressure at maximum flow (PdetQmax) of<20 cm H2O. Preoperative mean postvoid residual volume (PVR) was 16.1±32.3 mL, and the preoperative mean PdetQmax was 13.1±4.7 cm H2O. The postoperative PVR increased to 26.1±27.9 mL, which was not clinically significant. Their study showed an increase in PVR of greater than 100 mL in 7% of the patients, but the patients improved after 3 to 4 months of medical treatment with alpha-blockers. In another study evaluating the effect of TOT on the voiding phase, the success rate for SUI at mean follow-up of 76 months was similar for the DU group and the control group. However, voiding dysfunction after TOT significantly increased from 18% to 36% in the DU group, and de novo voiding dysfunction was significantly more common in the DU group [15]. In that study, DU was defined by a projected isovolumetric pressure index (PdetQmax+Qmax) of <30 cm H2O. The preoperative mean PdetQmax of the enrolled patients was 10.6±5.2 cm H2O. They reported that a PdetQmax of ≤12 cm H2O was a predictor for postoperative voiding dysfunction in patients with SUI and DU.
Ong and Kuo [4] reported that the outcome of retropubic MUS for SUI was not affected by preoperative bladder dysfunction. The patients with DU in that study had a preoperative mean PVR of 182.2±291.3 mL and had looser sling placement at the mid-urethra and adjustment of sling tension by a cough test at 300 mL of bladder filling. The 10-year continence rate in DU was 79.4%, which was not different from the outcomes of the patients with stable bladder or DO. Another study elucidating the outcome of retropubic MUS in SUI with DU showed that 76% of the patients had clinical improvement in their SUI, but 21% of the patients needed clean intermittent catheterization (CIC) to empty their bladder [16]. DU was defined by a PdetQmax of <20 cm H2O and a Qmax of <15 mL/sec in that study. The preoperative mean PVR was 186±150 mL, and the mean PdetQmax was 11.5 ±10.6 cm H2O. A preoperative Qmax of ≥6 mL/sec was a predictive factor for a successful outcome, whereas a Qmax of <6 mL/sec was a predictive factor for postoperative CIC.
Adjustable Sling Procedure
When patients with UAB and incontinence have risk factors for voiding dysfunction after surgery, conventional MUS allows for adjustment of the sling tension by loose sling placement during surgery. However, adjustment of the tension during surgery has limitations for various postoperative outcomes according to the patient’s preoperative condition. Adjustable slings are expected to have better outcomes than conventional MUS in patients with incontinence and UAB because they control tension both during and after surgery. Typically adjustable slings include transobturator adjustable tape (TOA) and the Remeex system.
Lee et al. [17] reported TOA as an effective modality for treating SUI with voiding dysfunction, which was defined as a Qmax of ≤12 mL/sec with a voided volume of ≥100 mL. Among patients with combined SUI and voiding dysfunction, the complete SUI cure rate was 76.7% and the satisfaction rate was 80% at 6-month follow-ups. There were no significant changes in Qmax and PVR. Only 1 patient required mesh cutting due to persistent postoperative voiding dysfunction. Jo et al. [18] showed the effects of TOA in patients with SUI and DU defined as Qmax of <15 mL/sec. The objective cure rate in SUI with DU was 80%, which was not significantly different from that of SUI only. There was no difference in the PVR in the UDS performed after surgery, but the Qmax decreased significantly from 17.0±9.4l mL/sec to 14.1±9.8 mL/sec in the SUI with DU group.
The Remeex system is an adjustable device that allows tension regulation at any time after surgery for SUI. A study evaluating Remeex system outcomes for patients with SUI and DU reported a treatment success rate of 81.5% over 38 months of follow-up and improved quality of life [19]. The Qmax decreased significantly after surgery from 12.6 ±6.3 mL/sec to 8.9±5.7 mL/sec, but the PVR did not change significantly, and persistent urinary retention was found in about 25% of the patients in that study. Chung and Yoo [20] investigated Remeex system outcomes for SUI with female voiding dysfunction, which was defined as a Qmax of ≤12 mL/sec when the voided volume was ≥150 mL. The subjective surgical outcomes and patient satisfaction were not significantly different for SUI with and without voiding dysfunction. The Qmax decreased and the PVR increased slightly in both groups after 2 months of followup, but these changes were not significantly different. Although the rate of tension regulation after surgery was significantly higher in the SUI with voiding dysfunction group, the complication rate was not significantly different between the 2 groups.
Surgical Treatment for Postprostatectomy Incontinence With UAB
About 40% of the patients with postprostatectomy incontinence (PPI) have DU due to bladder denervation during radical prostatectomy [21]. The main surgical treatments for PPI are artificial urinary sphincter (AUS) and male sling [22]. Some urologists are concerned about urinary retention after these surgical treatments for PPI with UAB. However, the outcomes and proportions of increased PVR after AUS for PPI were not different between voided by straining, implying impaired detrusor contractility and normal contractility [23,24]. The patients with PPI who require AUS implant have low urethral resistance and do not have high PVR before treatment for SUI. The possibility of complications such as voiding difficulty due to AUS may be low in patients who void normally.
The pressure change in the urethra during the voiding phase with a male sling can be small compared to the pressure changes in the urethra when a cuff is fully deflated during voiding with AUS. Thus, some argue that insufficient detrusor contractility cannot overcome the fixed resistance of a male sling during the voiding phase [25]. Han et al. [26] investigated the safety of male slings for PPI with impaired contractility, defined as a bladder contractility index less than 100 and insignificant PVR. Although the patients had impaired contractility, the preoperative mean PVR was 17.4 mL, and patients emptied their bladders well, with a maximum PVR of 140 mL. Patients with PPI and poor contractility who underwent sling procedure in that study had no difference in postoperative PVR and Qmax compared to the patients with normal contractility. Therefore, male slings can be used safely for PPI with UAB and normal urine emptying. However, there are insufficient studies on the outcomes of male slings in UAB patients with increased PVR and further research is necessary.
Role of Therapeutic Modalities for UAB in Incontinence
There are no proven standard treatment options for UAB. However, currently proposed UAB treatments, including behavioral, pharmacological, and CIC strategies can be applied when voiding symptoms due to UAB occur after treatment for incontinence.
Pharmacotherapy
Alpha-blockers relax smooth muscle within the bladder outlet and are effective for UAB-related bladder outlet obstruction (BOO). Alpha-blockers were also effective for LUTS and PVR in a study that enrolled patients with UAB but strictly excluded BOO patients [27]. Although surgical treatment such as tape incision or urethrolysis should be performed for persistent voiding dysfunction caused by obstruction after anti-incontinence surgery [28], alpha-blockers can be used for voiding dysfunction after anti-incontinence surgery related to preoperative UAB. However, alpha-blockers alone do not increase detrusor contractility in DU [29], and there is a lack of evidence regarding the efficacy of alpha-blockers in managing incontinence with UAB.
Parasympathomimetics can theoretically enhance detrusor contractility because they augment excitatory acetylcholine action between the synapses. However, parasympathomimetics are not recommended for UAB due to their potential adverse effects and unproven clinical efficacy [30]. Although parasympathomimetics such as bethanechol continue to be prescribed for UAB in elderly women [31], there is insufficient evidence for a role of bethanechol in UAB patients with incontinence.
Clean intermittent catheterization
The current mainstay of urinary retention in UAB is CIC. CIC can control incomplete bladder emptying and complications related to high PVR, but it is not a definitive treatment for UAB [32]. CIC should be recommended for urinary retention or when a patient cannot empty two-thirds of their bladder volume after anti-incontinence surgery [28]. CIC for voiding dysfunction after anti-incontinence surgery is conducted 3 to 4 times daily until the PVR decreases to less than 100 mL or is at most half of the voided volume [33]. Although the results may vary depending on the preoperative patient characteristics, more than 20% of the patients required CIC after a MUS in a study of SUI patients with DU who had more than 180 mL mean preoperative PVR [16]. Incontinence patients with UAB who can only empty their bladder through CIC are more likely to maintain CIC after incontinence treatment. Patients with neurogenic SUI related to spinal cord injury who can empty their bladder only by CIC had a high continent rate after implantation of an AUS, although CIC had to be maintained after surgery [34].
Future therapies
Since there are no definitive treatments for UAB, potential therapies such as stem cell therapy or gene therapy are in clinical trials for the management of UAB. Stem cell therapy for UAB is based on the improvement of detrusor contractility and voiding through regeneration of the detrusor smooth muscle [35]. Exogenous stem cell factor of neural and smooth muscle origin restored and improved detrusor contraction in rats with UAB by increasing the number of interstitial cells of Cajal [36]. The clinical efficacy and safety of intradetrusor injections of autologous muscle-derived cells for UAB were reported in a pilot study with humans [37]. Stem cells can restore the urethral sphincter function, and several clinical trials have shown the potential of stem cell therapy for managing SUI [38]. Although there is currently insufficient evidence, the application of stem cell therapy to both detrusor smooth muscle and urethral sphincter smooth muscle may be used to treat UAB patients with incontinence.
Gene therapy for UAB has been conducted experimentally using the concept of delivery genes that increase detrusor smooth muscle contractility. Goins et al. [39] reported that nerve growth factor gene delivery to sensory ganglia cells innervating the bladder using herpes simplex virus decreased the PVR in rats with neurogenic DU. Several studies investigating the genetic factors of SUI and developing future targeted therapies for SUI have reported the role of genes such as growth factor receptor-bound protein 2 [40]. Future research on gene therapy may be applicable to the treatment of incontinence with UAB.
Management of DHIC
The control of DHIC patients who have UUI and poor detrusor contractility is challenging. Since the DHIC is common in the elderly population who have multifactorial causes of lower urinary tract dysfunction, individual situations in patients should be considered when treating DHIC. DHIC patients who suffer from urinary retention need to catheterization for bladder emptying. However, in DHIC patients who showed UUI without urinary retention, other methods can be tried to control the symptoms.
Beta3-adrenergic receptor agonists that can relax the bladder without blocking of acetylcholine activity during detrusor contraction have been introduced for the management of DHIC with improvement in urgency and voiding efficiency [41]. However, there is a lack of evidence on the efficacy for UUI in DHIC yet. Intravesical onabotulinumtoxin A injection that is known to be effective for refractory OAB can be used in patients with DHIC. Although the subjective urgency symptom improved without an increased risk of adverse events, onabotulinumtoxin A has not shown efficacy for UUI in DHIC [42].
Sacral neuromodulation has the potential to control both UUI and compromised detrusor contractile function. Hennessey and Hoag reported that sacral neuromodulation for patients with DHIC could ameliorate OAB symptoms and decrease in PVR although they did not show an improvement in UUI due to the small patient numbers [43].
CONCLUSIONS
There is no definitive research establishing the most appropriate management for incontinence with UAB. The current surgical treatments for SUI may also be effective in patients with UAB. The results of trials for SUI with UAB are summarized in (Table 1). Since proper tension is important for treating SUI with UAB, adjustable slings may play an important role in patient management. In addition, other treatments for UAB may be applied to voiding dysfunction after incontinence treatment to improve patient voiding symptoms. The possibility of controlling UUI related to DHIC using sacral neuromodulation has also been consistently suggested. However, further research is needed to establish evidence for the efficacy and safety of treatments for incontinence with UAB and to improve patient quality of life.
Notes
Conflict of Interest
TH is an employee of Asahi Kasei Pharma Corporation. Others have no potential conflict of interests relevant to this article.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: JCK
· Formal Analysis: KJC
· Investigation: KJC
· Methodology: KJC
· Project Administration: JCK
· Writing–Original Draft: KJC
· Writing–Review & Editing: JCK