Alpha1-Adrenoceptor Antagonists Improve Memory by Activating N-methyl-D-Aspartate-Induced Ion Currents in the Rat Hippocampus
Article information
Abstract
Purpose:
Alpha1 (α1)-adrenoceptor antagonists are widely used to treat lower urinary tract symptoms. These drugs not only act on peripheral tissues, but also cross the blood-brain barrier and affect the central nervous system. Therefore, α1-adrenoceptor antagonists may enhance brain functions. In the present study, we investigated the effects of tamsulosin, an α1-adrenoceptor antagonist, on short-term memory, as well as spatial learning and memory, in rats.
Methods:
The step-down avoidance test was used to evaluate short-term memory, and an eight-arm radial maze test was used to evaluate spatial learning and memory. TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling) staining was performed in order to evaluate the effect of tamsulosin on apoptosis in the hippocampal dentate gyrus. Patch clamp recordings were used to evaluate the effect of tamsulosin on ionotropic glutamate receptors, such as N-methyl-D-aspartate (NMDA), amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA), and kainate receptors, in hippocampal CA1 neurons.
Results:
Tamsulosin treatment improved short-term memory, as well as spatial learning and memory, without altering apoptosis. The amplitudes of NMDA-induced ion currents were dose-dependently increased by tamsulosin. However, the amplitudes of AMPA- and kainate-induced ion currents were not affected by tamsulosin.
Conclusions:
Tamsulosin enhanced memory function by activating NMDA receptor-mediated ion currents in the hippocampus without initiating apoptosis. The present study suggests the possibility of using tamsulosin to enhance memory under normal conditions, in addition to its use in treating overactive bladder.
INTRODUCTION
Overactive bladder (OAB) syndrome is generally characterized by frequency, nocturia, and urgency [1]. OAB is commonly observed in clinical practice, and occurs in 16%–17% of adults worldwide. OAB, sometimes with unknown etiology, induces sustained high bladder pressure and causes urinary urgency [2]. Activation of alpha1 (α1)-adrenoceptors in the bladder causes outflow obstruction, bladder instability, and frequent micturition [3].
Antimuscarinics are commonly used to treat obstructive and voiding symptoms; however, these drugs have limited efficacy and are associated with side effects such as dry mouth, somnolence, constipation, and blurred vision [3]. A study of OAB in humans reported that daytime frequency, nocturia, and episodes of incontinence were decreased by treatment with α1-adrenoceptor antagonists [4]. Tamsulosin is classified as an α1-adrenoceptor antagonist and is widely used to reduce urinary obstruction and relieve associated manifestations in patients with symptomatic benign prostatic hyperplasia [5].
Glutamate is the primary excitatory neurotransmitter in the mammalian brain, and its actions are mediated by ionotropic and metabotropic glutamate receptors [6]. Glutamate is implicated in many physiological processes, including learning and memory [7-10]. Moreover, glutamate and its receptors are involved in the proliferation, migration, differentiation, and survival of neuronal progenitor cells [11]. Glutamate binds postsynaptically to three ionotropic glutamate receptors: N-methyl-D-aspartate (NMDA) receptors, amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and kainate receptors. Ionotropic glutamate receptors mediate the excitatory neurotransmission that is crucial for various aspects of brain development and function, including learning and memory formation [12]. Additionally, ionotropic glutamate receptors in the hippocampus affect synaptic strength and the capacity for plasticity, as well as contributing to spatial learning and memory [13].
Apoptosis is a form of cell death that constitutes a common mechanism in cell replacement, tissue remodeling, and damaged cell removal. Apoptosis is triggered by a variety of pathological stimuli [14]. Blocking α1-adrenoceptors facilitates the recovery of memory functions by inhibiting hippocampal cell apoptosis and proliferation, which demonstrates the neuroprotective effect of tamsulosin against intracerebral hemorrhage [15]. Apoptotic cell death can be assessed using terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL) staining, which detects DNA fragmentation [16].
Many α1-adrenoceptor antagonists, including tamsulosin, are used to treat OAB symptoms. Tamsulosin crosses the blood-brain barrier and may impact OAB-induced micturition dysfunction by inhibiting neuronal activation in the voiding centers [17,18]. However, the beneficial effects of α1-adrenoceptor antagonists on learning and memory remain unknown. In the present study, the effects of tamsulosin on short-term memory and on spatial learning and memory were assessed in rats. The effects of tamsulosin on ionotropic glutamate-receptor-mediated currents were measured using the nystatin-perforated patch clamp technique. In addition, we examined whether tamsulosin initiates apoptosis in neurons, using TUNEL staining.
MATERIALS AND METHODS
Experimental Animals and Treatments
All experiments were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences. Sprague-Dawley female rats (7 weeks old; weight, 210±10 g)were randomly divided into the following 5 groups (n=8 in each group): control group, 0.001-mg/kg tamsulosin-treated group, 0.01-mg/kg tamsulosin-treated group, 0.1-mg/kg tamsulosin-treated group, and 1.0-mg/kg tamsulosin-treated group.
The rats in the tamsulosin-treated groups received oral tamsulosin (Harnal, Astellas Pharma Inc., Tokyo, Japan) once each day for 4 consecutive weeks, at the doses listed above. The animals in the control group received an equivalent dose of distilled water once each day for 4 consecutive weeks.
Step-Down Avoidance Test
The latency time in the step-down avoidance test was measured according to previously described methods, in order to evaluate short-term memory [19,20]. Training in the step-down avoidance test began 28 days after starting tamsulosin treatment. The rats were placed on a 7×25-cm platform that was 2.5 cm high. The platform faced a 45×25-cm grid of parallel 0.1-cm caliber stainless steel bars, spaced 1 cm apart. In the training session, the rat received a 0.2-mA scrambled foot shock that lasted for 2 seconds and began immediately upon stepping down. Two hours after the training session, the latency time (second), which was defined as the interval between the rats stepping down on the platform and placing all four paws on the grid, was measured in each group. A latency time over 180 seconds was scored as 180 seconds.
Eight-Arm Radial Maze Test
Spatial learning and memory were examined using an eight-arm radial maze test, according to previously described methods [19,21]. The radial-arm maze apparatus consisted of a central octagonal plate (30 cm in diameter) and eight radiating arms (50 cm long and 10 cm wide). The apparatus was placed 1 m above the floor. A small receptacle filled with water (3 cm in diameter and 1 cm in depth) was located at the end of one arm. The rat was trained in the maze three times before beginning an experimental test. Training sessions were conducted 21, 24, and 26 days after starting tamsulosin treatment. During the training sessions, the rat was deprived of water for 24 hours and allowed to search for the water in the maze for 5 minutes after finishing each session. The test was conducted 27 days after starting tamsulosin treatment. The duration of time each rat spent seeking water at the end of the arms was recorded. The test was terminated when the rat found the water in all eight arms, or when >8 minutes elapsed. The number of correct choices before the first error was counted, and re-entry into a previously visited arm was counted as an error.
Tissue Preparation
The rats were sacrificed immediately after determining the latencies in the step-down avoidance test. Rats were anesthetized using Zoletil 50 (10 mg/kg, intraperitoneal injection; Virbac Laboratories, Carros, France), transcardially perfused with 50mM phosphate-buffered saline, and fixed with a freshly prepared solution consisting of 4% paraformaldehyde in 100mM phosphate buffer (pH 7.4). The brains were removed, stored overnight in fixative, and then transferred to 30% sucrose for cryoprotection. For the TUNEL staining, 40-μm thick coronal sections were prepared using a cryostat (Leica, Nussloch, Germany). On average, 10 sections were collected from the hippocampal dentate gyrus of each rat.
TUNEL Staining
In order to visualize DNA fragmentation using a marker for apoptosis, TUNEL staining, was performed using an In Situ Cell Death Detection Kit (Roche, Mannheim, Germany), according to the manufacturer’s protocol [10,22]. The sections were postfixed in ethanol-acetic acid (2:1) and rinsed. They were then incubated with proteinase K (100 μg/mL), rinsed, incubated in 3% H2O2, permeabilized with 0.5% Triton X-100, rinsed again, and incubated in the TUNEL reaction mixture. Next, the sections were rinsed and visualized using Converter-POD with 0.03% 3,3´-diaminobenzidine. Mayer’s hematoxylin (DAKO, Glostrup, Denmark) was used as a counter-stain, and the sections were mounted on gelatin-coated slides. The slides were air-dried overnight at room temperature, dehydrated through a gradient of ethanol, and coverslipped using Permount (Fisher Scientific, New Jersey, NJ, USA).
Preparation of Hippocampal CA1 Neurons
Hippocampal CA1 neurons were dissociated using a previously described technique [23,24]. In brief, Sprague-Dawley rats of both sexes were decapitated under Zoletil 50-induced anesthesia (50 mg/kg, intramuscular injection) when they were 10–15 days old. The brains were removed, and transverse slices (400 µm thick) were made with a microslicer (DTK-1000, DSK, Tokyo, Japan). The slices were preincubated in an oxygenated solution (95% O2 and 5% CO2) at room temperature for 30 minutes. The slices were then treated with pronase (protease XIV, 1 mg/6 mL of the oxygenated incubation solution) for 40–60 minutes at 32°C, and subsequently treated with thermolysin (protease X, 1 mg/6 mL) for 10–20 minutes at 32°C. After the enzyme treatment, the slices were incubated in an enzyme-free incubation solution for 1 hour.
The hippocampal CA1 region was identified under a binocular microscope (SZ-ST, Olympus, Tokyo, Japan), and isolated from the slices with an electrolytically polished injection needle. The isolated hippocampal CA1 neurons were mechanically dissociated with fire-polished fine glass Pasteur pipettes in 35-mm plastic culture dishes (Falcon, Franklin Lakes, NJ, USA) filled with standard solution. The dissociation procedure was performed under an inverted phase-contrast microscope (CK-2, Olympus). The dissociated neurons usually adhered to the bottom of the dish within 20 minutes. These cells remained viable for electrophysiological studies for a maximum of 6 hours after dissociation.
Solutions
The ionic composition of the incubation solution was (in mM): NaCl 124, KCl 5, KH2PO4 1.2, MgSO4 1.3, CaCl2 2.4, glucose 10, and NaHCO3 24. The pH was adjusted to 7.4 by continuous bubbling with 95% O2 and 5% CO2. The composition of the standard external solution was (in mM): NaCl 150, KCl 5, MgCl2 1, CaCl2 2, glucose 10, and N-2-hydroxyethylpiperazine-N´-2-ethanesulphonic acid (HEPES) 10. The pH was adjusted to 7.4 using tris-(hydroxymethyl)aminomethane (Tris-base). The composition of the internal pipette solution for nystatin-perforated patch recordings was (in mM): KCl 150 and HEPES 10. The pH was adjusted to 7.2 using Tris-base. A stock solution containing 10 mg/mL nystatin was prepared and added to the internal patch pipette solution in order to reach a final concentration of 200 µg/mL.
Electrophysiological Measurements
Electrophysiological recordings were performed using the nystatin-perforated patch recording mode under a voltage clamp condition, according to previously described methods [23,25]. Patch pipettes were prepared from glass capillaries with an outer diameter of 1.5 mm, using a two-stage pipette puller (PB-7, Narishige, Tokyo, Japan). The resistance between the recording electrode filled with the internal pipette solution and the reference electrode was 6–8 MΩ. After forming a stable perforated patch, the series resistance ranged from 16–25 MΩ. Electrical stimulation, current recordings, and current filtration (at 2.9 kHz) were obtained with an EPC-7 patch clamp amplifier (List-Electronic, Darmstadt/Eberstadt, Germany). The current and voltage were also monitored with a pen recorder (Recti-Horiz-8K, NEC San-ei, Tokyo, Japan). All experiments were performed at room temperature (22°C–24°C).
Data Analysis
The data were analyzed with one-way analysis of variance and then Duncan post hoc tests. All values are expressed as the mean±standard error of the mean, and P-value<0.05 was considered significant.
RESULTS
Effect of Tamsulosin on Short-Term Memory Using the Step-Down Avoidance Test
The results of the analysis examining the effect of tamsulosin on the latency time on the step-down avoidance test are presented in Fig. 1. Treatment with tamsulosin dose-dependently increased the latency time (P<0.05). These results demonstrate that treatment with tamsulosin for four weeks improved short-term memory in rats.

Effect of tamsulosin on short-term memory in the step-down avoidance test. Control group (CON), 0.001-mg/kg tamsulosin-treated group (T-0.001), 0.01-mg/kg tamsulosin-treated group (T-0.01), 0.1-mg/kg tamsulosin-treated group (T-0.1), and 1-mg/kg tamsulosin-treated group (T-1). The data are presented as the mean±standard error of the mean. *P<0.05 compared to the control group. †P<0.05 compared to the 0.1-mg/kg tamsulosin-treated group.
Effect of Tamsulosin on Spatial Learning and Memory in the Eight-Arm Radial Maze Test
The duration of time for rats to complete a performance, the number of correct choices before the first error, and the number of errors before eight successful performances in the eight-arm radial maze test are presented in Fig. 2. Treatment with tamsulosin decreased the duration of time to complete a performance, the number of errors and increased the number of correct choices (P<0.05). Therefore, the results demonstrate that treatment with tamsulosin for four weeks improved spatial learning and memory in rats.

Effect of tamsulosin on spatial learning in the eight-arm radial maze test. Control group (CON), 0.001-mg/kg tamsulosint-reated group (T-0.001), 0.01-mg/kg tamsulosin-treated group (T-0.01), 0.1-mg/kg tamsulosin-treated group (T-0.1), and 1-mg/kg tamsulosin-treated group (T-1). The data are presented as the mean±standard error of the mean. *P<0.05 compared to the control group.
Effect of Tamsulosin on the Number of TUNEL-Positive Cells in Hippocampal Dentate Gyrus
Photomicrographs of TUNEL-positive cells in the hippocampal dentate gyrus are presented in Fig. 3A. Treatment with tamsulosin did not alter the number of TUNEL-positive cells (Fig. 3B). These results demonstrate that treatment with tamsulosin for four weeks did not initiate apoptosis in the hippocampus.
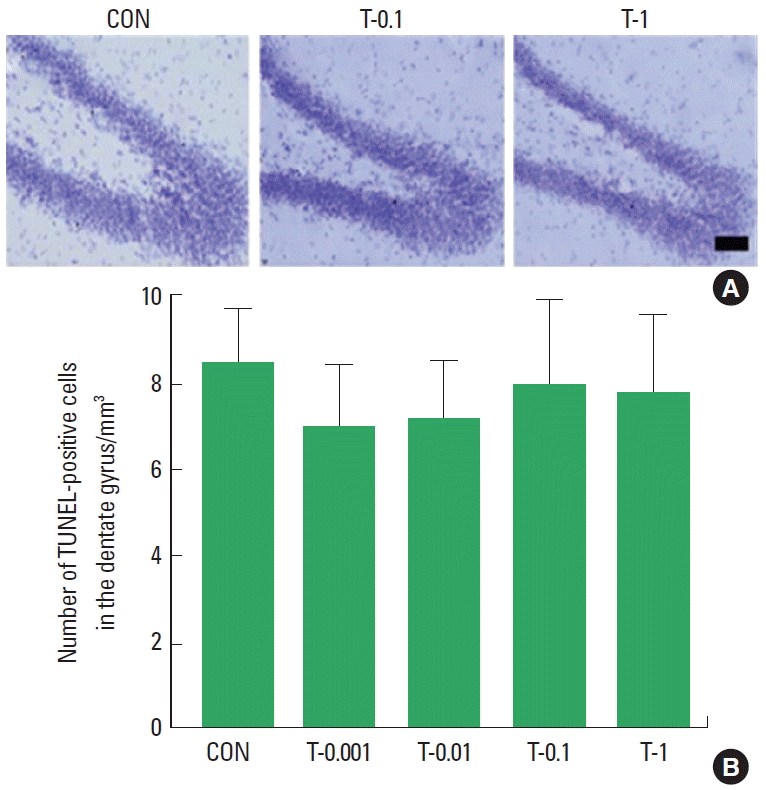
Effects of tamsulosin on DNA fragmentation. (A) Photomicrographs of terminal deoxynucleotidyl transferase-mediated dUTP nick end labeling (TUNEL)-positive cells in the hippocampal dentate gyrus. (B) The number of TUNEL-positive cells in each group. Scale bar=100 μm. Control group (CON), 0.001-mg/kg tamsulosin-treated group (T-0.001), 0.01-mg/kg tamsulosin-treated group (T-0.01), 0.1-mg/kg tamsulosin-treated group (T-0.1), and 1-mg/kg tamsulosin-treated group (T-1). The data are presented as the mean±standard error of the mean.
Effect of Tamsulosin on the NMDA-Induced Ion Current
The application of NMDA dose-dependently evoked inward currents (Fig. 4A, B). The magnitude of the ion currents induced by 100µM NMDA alone was used as the control value. The initial amplitude of the NMDA-induced ion current varied by less than 5% during the recording period prior to tamsulosin application. The magnitude of the NMDA-induced ion current was dose-dependently enhanced by coapplication of tamsulosin (P<0.05) (Fig. 4C, D). These results demonstrate that tamsulosin potentiated the NMDA-induced ion current in hippocampal CA1 neurons.

Effect of tamsulosin on the N-methyl-D-aspartate (NMDA)-induced ion current in hippocampal CA1 neurons. (A) Typical traces of currents induced by increasing the concentrations of NMDA. (B) Concentration-response curve of NMDA-induced ion current. (C) Representative traces of 100μM NMDA-induced current with the application of 0.1μM, 1μM, and 3μM tamsulosin. (D) Concentration-dependent effect of tamsulosin on the NMDA-induced ion current. *P<0.05 compared to the 100μM NMDA-induced ion current. †P<0.05 compared to the 100μM NMDA-induced ion current with 0.1μM tamsulosin.
Effect of Tamsulosin on the AMPA-Induced Ion Current
The application of AMPA dose-dependently evoked inward currents (Fig. 5A). The magnitude of the ion current induced by 100µM AMPA alone was used as the control value. The initial amplitude of the AMPA-induced ion current varied by less than 5% during the recording period. The magnitude of the AMPA-induced ion current was not altered by coapplication of 0.3µM tamsulosin (Fig. 5B, C). Therefore, the results demonstrate that tamsulosin did not exert a significant effect on the AMPA-induced ion current in hippocampal CA1 neurons.
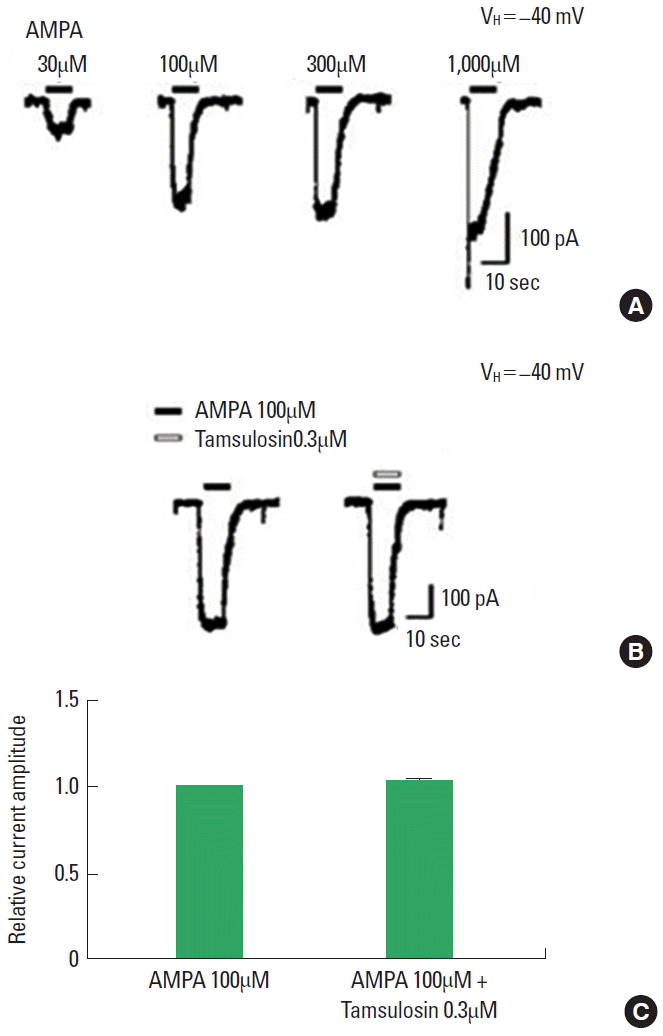
Effect of tamsulosin on the amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)-induced ion current in hippocampal CA1 neurons. (A) Typical traces of currents induced by increasing concentrations of AMPA. (B) Representative trace of a 100μM AMPA-induced current with application of 0.3μM tamsulosin. (C) Histogram showing the mean±standard error of the mean.
Effect of Tamsulosin on the Kainate-Induced Ion Current
The application of kainate dose-dependently evoked inward currents (Fig. 6A). The magnitude of the ion current induced by 100µM kainate alone was used as the control value. The initial amplitude of kainate-induced ion currents varied by less than 5% during the recording period. The magnitude of the kainate-induced ion current was not altered by coapplication of 0.3µM tamsulosin (Fig. 6B, C). The results demonstrate that tamsulosin did not have a significant effect on AMPA-induced ion currents in hippocampal CA1 neurons.
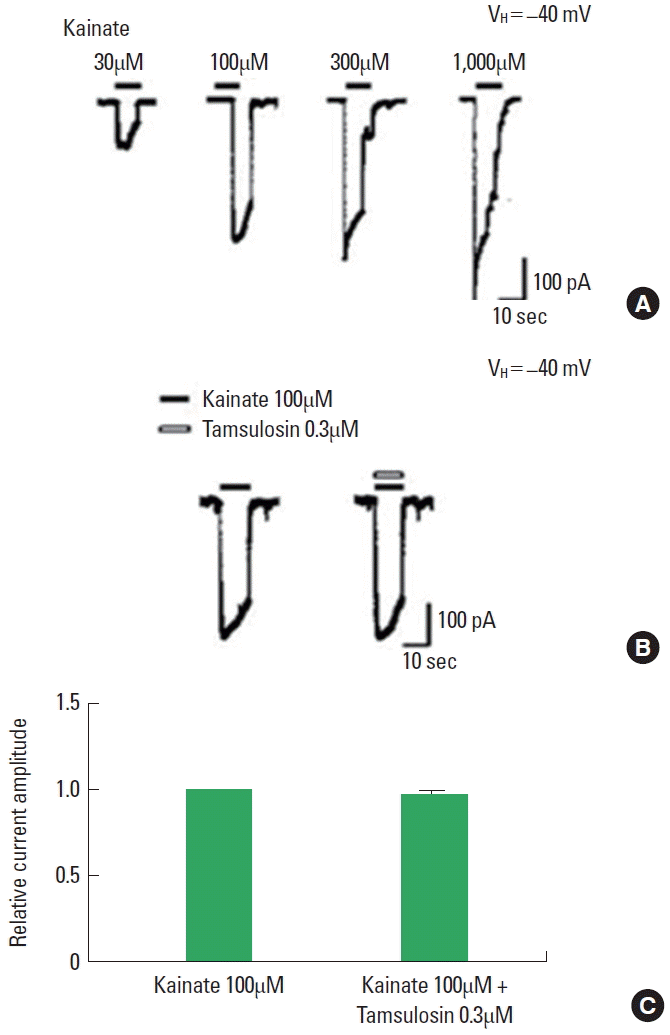
Effect of tamsulosin on the kainate-induced ion current in hippocampal CA1 neurons. (A) Typical traces of currents induced by increasing concentrations of kainate. (B) Representative traces of 100μM kainate-induced ion current with the application of 0.3μM tamsulosin. (C) Histograms showing the mean±standard error of the mean.
DISCUSSION
We hypothesized that the activity of tamsulosin on the central nervous system would result in memory improvements in rats. This hypothesis was based on several previous studies demonstrating that tamsulosin crosses the blood-brain barrier [18], activation of α1-adrenoceptors inhibits memory consolidation [26], and overexpression of α1-adrenoceptors produces apoptotic neurodegeneration [27]. Memory improvements by tamsulosin are likely associated with activation of ionotropic glutamate receptors by glutamate, which previous research has demonstrated as a mechanism of learning and memory [7-10]. Using the step-down avoidance task and an eight-arm radial maze task, we found that treatment with tamsulosin for four weeks improved short-term memory, as well as spatial learning and memory, respectively, in rats. However, TUNEL staining demonstrated that tamsulosin treatment did not initiate apoptosis in the hippocampus. Patch clamp recordings indicated that tamsulosin potentiated the NMDA-induced ion current, but not the AMPA- or kainate-induced currents, in hippocampal CA1 neurons. Collectively, our results demonstrate that tamsulosin enhanced memory by activating the NMDA receptor-mediated ion current in the hippocampus, without initiating apoptosis.
Previous studies have investigated α1-adrenoceptor antagonists as treatments for lower urinary tract symptoms. For example, tamsulosin has the potential to overcome OAB-induced micturition dysfunction by inhibiting neuronal activation in the voiding centers [17]. Improved memory was an unexpected physiological consequence of tamsulosin treatment, and it has been hypothesized that activity on the central nervous system is the mechanism for this effect. However, a study examining α1-adrenoceptor antagonists for treatment of central nervous system lesions has not been conducted.
Adrenoceptors are classified as α1-, α2-, and β-adrenoceptors, and comprise the family of heterotrimeric G-protein-coupled receptors that are activated by epinephrine and norepinephrine [25]. Previous research has found that α1-adrenoceptors are involved in the regulation of epileptiform activity [28], long-term synaptic depression [29], and attention and cognitive processes [30]. Several previous studies have also investigated the function of α1-adrenoceptors. Arnsten et al. [31] reported that stimulating α1-adrenoceptors impaired prefrontal cortical cognitive function. Activation of α1-adrenoceptors in the chick forebrain by the α1-adrenoceptor agonist methoxamine inhibited memory consolidation [26]. In the present study, tamsulosin, an α1-adrenoceptor antagonist, increased the latency time of rats in the step-down avoidance test, indicating that tamsulosin improved short-term memory (Fig. 1). Tamsulosin treatment also shortened the performance time, increased the number of correct choices, and decreased the number of error choices in the eight-arm radial maze test, suggesting that tamsulosin enhanced spatial learning and memory (Fig. 2). Collectively, these results demonstrate that tamsulosin improves memory under normal conditions.
The memory improvement induced by tamsulosin is likely related to the neuroprotective effects of α1-adrenoceptor-mediated inhibition of apoptosis. Zuscik et al. [27] demonstrated that overexpression of α1-adrenoceptors resulted in apoptotic neurodegeneration with corresponding multiple-system atrophy. A previous study demonstrated that tamsulosin exerted a neuroprotective effect on intracerebral hemorrhage by inhibiting hippocampal cell apoptosis via α1-adrenoceptor blockade [15]. During the apoptotic process, the number of TUNEL-positive cells increases [22], whereas an unchanged number of TUNEL-positive cells indicates that apoptosis did not occur [32]. In the present study, the number of TUNEL-positive cells in the hippocampus did not change following tamsulosin treatment (Fig. 3). These results confirmed that tamsulosin does not initiate apoptotic neuronal cell death.
There appears to be a relationship between tamsulosin and glutamate, as well as between tamsulosin and ionotropic glutamate receptors, which participate in the mechanisms of learning and memory, particularly when apoptotic neuronal cell death does not occur. Glutamate is a major excitatory neurotransmitter in the central nervous system, and contributes to numerous physiological and pathological processes [7]. The excitatory action of glutamate is particularly prominent in the hippocampus, outer layer of the cerebral cortex, and substantia gelatinosa of the spinal cord. Excitatory neurotransmission initiated by glutamate is involved in many physiological processes, including learning and memory [33]. Subtypes of the glutamate receptor are implicated in diverse brain functions. For example, NMDA receptors are involved in activity-dependent synaptic plasticity, such as long-term potentiation [10]. Increasing the expression of NMDA receptors has alleviated schizophrenia-related symptoms and improved memory function [34], and inhibition of NMDA receptors in the hippocampus is accompanied by impaired spatial learning and memory, as well as locomotion activity [35].
In the present study, tamsulosin application to hippocampal neurons increased the amplitude of NMDA-induced ion currents. AMPA receptor endocytosis mediates the decay of long-term potentiation, while inhibiting this process can prolong long-term potentiation [8]. We found that tamsulosin application did not exert any effects on the amplitude of AMPA-induced ion currents. In addition to NMDA and AMPA receptors, kainate receptors have also been linked to spatial learning and memory, as well as excitotoxic neurodegeneration [9]. However, tamsulosin did not exert any effects on the amplitude of kainite-induced ion currents in the present study. Collectively, these results demonstrate that tamsulosin only activates the NMDA-induced ion current in the hippocampus.
The present study demonstrated that tamsulosin improves memory by activating NMDA receptors under normal conditions. Tamsulosin is typically prescribed to geriatric patients, due to the correlation between aging and urinary voiding conditions. Thus, in vivo findings with similar results, may contribute to development of new methods to improve memory in the elderly, thereby using tamsulosin to improve their quality of life.
Notes
Grant Support
The study was supported by a grant from the National Research Foundation of Korea (grant No.: NRF-2013R1A1A1063509).
Research Ethics
All experiments were performed in accordance with the animal care guidelines of the National Institutes of Health and the Korean Academy of Medical Sciences.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
