Circadian Rhythms in Urinary Functions: Possible Roles of Circadian Clocks?
Article information
Abstract
Circadian clocks are the endogenous oscillators that harmonize a variety of physiological processes within the body. Although many urinary functions exhibit clear daily or circadian variation in diurnal humans and nocturnal rodents, the precise mechanisms of these variations are as yet unclear. In this review, we briefly introduce circadian clocks and their organization in mammals. We then summarize known daily or circadian variations in urinary function. Importantly, recent findings by others as well as results obtained by us suggest an active role of circadian clock genes in various urinary functions. Finally, we discuss possible research avenues for the circadian control of urinary function.
INTRODUCTION
One of the most predictable environmental changes on this rotating planet is undoubtedly the alternations of day and night, which accompany daily variations in environmental illumination, ambient temperature, humidity, and food and predator availability. Organisms that can predict, and prepare themselves in advance for, these environmental changes have selective advantages over those who cannot accommodate themselves until the changes of environment have taken place. Indeed, almost every life form on Earth displays distinct daily or circadian variations in biochemistry, physiology, pathology, and behavior [1-4].
The advent of endogenous circadian clocks seems to be a recent innovation in the history of life, because the core molecules that derive oscillatory functions within the cell have multiple origins among different forms of life [1]. Gehring and Rosbash [5] once hypothesized that the origin of circadian clocks in early metazoans might be a coupling of blue light receptors to molecular oscillators to avoid excess oxidative stress induced by light [6]. In support of this notion, the phases of DNA replication, DNA repair, and the cell cycle are found appropriately positioned in a given day-night cycle to minimize the damage [7,8]. Moreover, disturbances to the circadian rhythm and knockouts of clock genes lead to various oxidative stress-related pathologies, including mental illnesses, cancer, obesity, type II diabetes, cardiovascular diseases, and aging [2,4,9-14]. Thus, it has become increasingly evident that circadian clocks play crucial roles in the orchestration of life according to time of day, and most physiological functions are tightly locked to an organism's activity and rest phases to ensure optimal performance.
Like many other physiological processes, fluid intake, urine production, and urine storage display distinct daily and circadian variations as discussed below. Yet, the precise mechanism or mechanisms underlying these variations remain largely unexplored. In this review, we briefly introduce circadian systems in mammals and their physiological significances, keeping urological researchers in mind. Then, we explore the known daily and circadian variations in urinary functions. Finally, we discuss future areas of research in terms of circadian control of urinary functions.
THE CIRCADIAN ORGANIZATION IN MAMMALS
Circadian rhythms are defined not by the molecular mechanisms underlying them but by well-established experimental criteria. For a process to be defined as a circadian behavior, three conditions should be met [15-17]. First, a rhythm must persist with a period of approximately 24 hours, even in the absence of an environmental cycle. The period length observed under constant environmental conditions constitutes the free-running period of a rhythm. Second, the period should vary only slightly at different ambient temperatures within the organism's physiological range. This criterion underlies the temperature compensation of a rhythm that mainly reflects the ability to maintain similar period lengths despite various factors affecting the velocity of biochemical and metabolic reactions, such as body temperature and nutritional status. Finally, the phasing of peaks and troughs relative to a reference point can be reset by environmental cues to which the rhythm entrains. This phase-resetting property allows organisms to accommodate themselves, for example, to the seasonal variations in environmental illumination. The environmental factors that can reset the phase of a rhythm are known as zeitgebers. Some important zeitgebers in mammals include light-dark cycles, temperature cycles, food availability, physical activity, and social cues [16-20].
Conceptually, three basic elements are needed for the proper operation of circadian systems in whole animals. First of all, a functional oscillator or pacemaker is needed that gives the basic drumbeat and sets the period of a rhythm. The suprachiasmatic nucleus (SCN) is now well defined as the master oscillator in mammals [21,22]. Experimental lesions in the SCN lead to an immediate loss of rhythmic behaviors [23], whereas persistence of circadian rhythmicity is observed in hypothalamic "islands" containing the SCN [24]. Moreover, fetal SCN transplants restore circadian rhythmicity in SCN-lesioned animals [25] and even determine the period of locomotor activity rhythms of the host animals [26]. The mammalian SCN can be divided into two parts: ventrolateral vs. dorsomedial. The ventrolateral SCN is composed of sparse spherical neurons with organelle-rich cytoplasm that synthesize vasoactive intestinal peptide, peptide histidine isoleucine, and gastrin-releasing peptide. The dorsomedial part of the SCN contains small elongated neurons with large nuclei and few cell organelles that mainly express vasopressin [21,22].
The second element of a circadian system is the presence of input pathways that can correct the non-24-hour basic periodicity of the central pacemaker to 24 hours and adjust the phase to local time. It is now well established that the SCN can be entrained by light-dependent signals via the retinohypothalamic tract [27-30]. A recent study demonstrated that the RHT, one component of the optic nerve, transmits the environmental light signal to four major fields in the hypothalamus: ventrolateral SCN, perisuprachiasmatic region, ventromedial part of the anterior hypothalamus, and ventral zone of the anterior group of the lateral hypothalamic area [29]. Other input pathways to the SCN include neuropeptide Y input from the intergeniculate leaflet via the geniculohypothalamic tract and serotonergic input from the Raphe nuclei [22,31-33]. Moreover, humoral feedback inputs to the SCN are believed to modulate the SCN clock nonphotically. For example, the SCN is one place that contains the highest concentration of melatonin receptors within the body [22,34]. Thus, the mammalian SCN receives information from the environment as well as from within the body and can adjust the relevant physiological rhythms accordingly.
The final element of a circadian system is the neural/humoral output pathways that transfer the rhythmic messages to effector organs of the overt rhythms. Major neural output pathways from the mammalian SCN include the subparaventricular zone, dorsomedial nucleus and medial preoptic area of the hypothalamus, and paraventricular nucleus of the thalamus [22,33,35-37]. In addition, virtually all hormones are secreted rhythmically depending on the time of day [20,38]. Indeed, the SCN is believed to play central roles in the circadian control of neuroendocrine functions [39]. In this way, the neural and humoral outputs derived from the central pacemaker govern the circadian rhythms of physiology and behavior.
GENETIC BASIS OF CIRCADIAN RHYTHMS: THE MOLECULAR OSCILLATOR WITHIN THE CELL
In 1971, Konopka and Benzer [40] reported the first clock mutants of Drosophila. The affected gene, named as period, was subsequently cloned [41]. Likewise, the first clock mutant mouse was reported by Vitaterna et al. [42]. Since then, several core clock genes that govern circadian rhythmicity in mammals have been identified [1-4]. These include circadian locomotor output cycle kaput (Clock), neuronal PAS domain protein 2 (Npas2), brain and muscle arnt-like 1 (Bmal1), mammalian homologues of Drosophila period (Per1 and Per2), and two cryptochromes (Cry1 and Cry2). Within the cell, the positive circadian transcription factor complex CLOCK (or NPAS2)/BMAL1 binds to the E-box sequences and transactivates the transcription of target genes, including pers and crys genes (Fig. 1). PERs and CRYs accumulate in the cytoplasm, translocate into the nucleus with a timed delay, and inhibit their own transcription by interacting with the CLOCK/BMAL1 complex [43]. Although this constitutes the primary feedback loop, there exists a second feedback loop involving the Rev-erba gene [44]. REV-ERBα, also transactivated by CLOCK/BMAL1, acts as a strong repressor of the Bmal1 gene, giving robustness to the molecular oscillator.
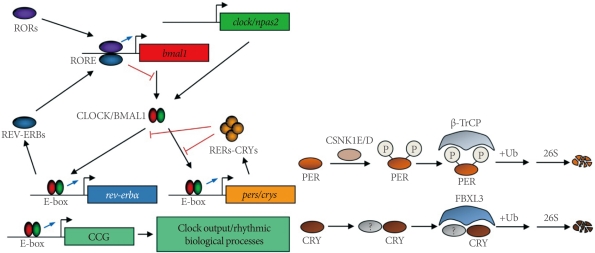
A current working model of the molecular oscillator in mammals. RORs, RAR-related orphan receptors; RORE, ROR response element; bmal1, brain and muscle arnt-like 1; clock, circadian locomotor output cycle kaput; npas2, neuronal PAS domain protein 2; CCG, clock-controlled gene; CSNK1E/D, casein kinase 1 epsilon/delta; β-TrCP, β-transducin repeat-containing protein; FBXL3, F-box and leucine-rich repeat protein 3.
The characterization of the tau mutant hamster, which harbors an A178C missense mutation in the casein kinase 1 epsilon (Csnk1e) gene, has emphasized the role of posttranslational modifications of circadian clock proteins [45,46]. It has subsequently been shown that β-TrCP and FBXL3 E3 ligase complexes target PERs and CRYs for degradation [47-50]. More recently, AMPK was shown to phosphorylate CRY1 and destabilize it in response to nutrient signals in the mouse liver [51]. Also notable is the chromatin remodeling and other modifications of circadian clock proteins that alter circadian gene expression and function [52-55].
TISSUE-SPECIFIC FUNCTIONS DRIVEN BY PERIPHERAL CLOCKS
Since the discovery of the circadian clock genes, one of the most striking findings was that circadian gene expression is not confined to the central oscillator in the SCN: circadian genes are expressed in almost every tissue within the body. Even cultured cells in vitro exhibit a robust oscillation of circadian genes in a self-sustained and cell-autonomous manner [56,57], thus allowing the characterization of the regulation and function of circadian genes in vitro [58-60].
Although almost every cell and tissue expresses circadian genes, cycling transcripts are not the same among the tissues or cells. For example, the SCN and the liver have only small portions of cycling transcripts in common [61]. Almost ubiquitous expression of clock genes and cell- and tissue-specificity in the cycling transcripts lead to the concept of peripheral clocks, which is believed to derive tissue-specific functions according to time of day [62-67]. Thus, the hierarchical organization of the central clock in the SCN and local peripheral oscillators in the peripheral tissues seem to generate the harmony of various physiological rhythms in the mammalian circadian timing system.
DAILY/CIRCADIAN RHYTHMS IN URINARY FUNCTION
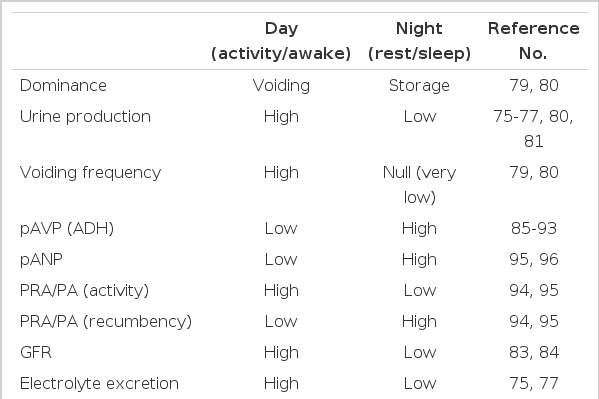
With the effective circadian timing system, most physiological functions in mammals are tightly locked to their activity and rest phases to ensure optimal performance. For example, diurnal animals consume most of their food and water during the day, whereas nocturnal animals do so during the night [20,68]. Accordingly, water intake, urine production, and urine storage also display distinct daily or circadian variations in diurnal humans and nocturnal rodents (Table 1). As discussed briefly in the previous issue [69], urine production and voiding must predominate during the active phase, whereas increased storage of urine in the bladder and reduced frequency in urination must be ensured to promote better rest and sleep. Consequently, disruption of this homeostatic regulation is predicted to decrease an organism's well-being. For example, nocturia, the complaint that an individual has to wake at night one or more times to void [70], decreases quality of life and negatively affects morbidity and mortality, especially in the elderly [71-74].
Diurnal or circadian variations in urine volume, electrolyte excretion, uroflow, micturition frequency, volume per void, and urine and osmole output rates have been extensively studied [75-82]. For example, water and electrolyte excretion is significantly low during the sleep phase compared with active daytime in healthy control subjects but not in patients with congestive heart failure or cirrhosis of the liver [75]. Moreover, these variations in urinary excretion are observed not only in nychthemeral conditions but also in constant-routine protocols [77], indicating that these rhythms are generated endogenously. Increased urine volume, urine production rate, and frequency during the day along with increased bladder capacity during the night [79,80] substantiates the dominance of voiding during the day with the dominance of urine storage during the night. Interestingly, maximum voided volume in school-aged children with monosymptomatic nocturnal enuresis was shown to be smaller than that in age-matched controls, but the maximum volume obtained after a standard holding exercise was not significantly different from that in the control group, which raises the possibility that there could be a defect in circadian rhythm for detrusor inhibition [81,82].
One of the well-known circadian rhythms in humans is the rhythm of glomerular and tubular function. Independent of circulating hormones, the glomerular filtration rate decreases by 15 to 30% overnight [83,84]. Nonetheless, many circulating hormones greatly affect urine production and storage. Of these, arginine vasopressin (AVP) displays a distinct diurnal/circadian variation and has been implicated in many urological problems, and its synthetic analog desmopressin has been widely used as a therapeutic agent [85-93]. Importantly, alterations or dampening of diurnal rhythms in the plasma AVP level have been linked to many urological problems including urological aging [86,87,90,91,93], further emphasizing the importance of proper circadian control in hormone secretion. Other urinary function-related hormones that exhibit diurnal or circadian fluctuations include plasma renin, aldosterone, atrial natriuretic peptide, cortisol, and prostaglandins [94-97] (Table 1). Thus, the harmonious secretion of these hormones may contribute to the distinct circadian variation in urinary functions.
Also notable is the daily variation in sympathetic/parasympathetic tone [98], considering the importance of the autonomic nervous system in bladder function. However, increased sympathetic tone during the active phase is quite contradictory to the notion that urine storage must predominate during the rest period. Yet, local bladder control of the autonomic nervous system still remains an intriguing possibility. Finally, the circadian control of blood pressure needs to be considered when examining urinary rhythms, because electrolyte excretion plays crucial roles in the control of blood pressure, and the dysregulation of blood pressure has wide significance for many human pathologies [99-101].
ROLES OF CIRCADIAN CLOCKS IN URINARY FUNCTION?
Despite the ample evidence supporting clear circadian and diurnal variations in urine production and storage, the mechanism or mechanisms underlying this variation are largely unknown. Do circadian clocks, especially the local clocks in the kidney and bladder if they exist, have any significant roles? Two recent studies support this possibility. Zuber et al. [102] demonstrated a local renal clock that may derive time-of-day-dependent renal functions. In their study, clock -/- mice exhibited a complex phenotype with partial diabetes insipidus, dysregulation of sodium excretion rhythms, and a significant decrease in blood pressure [102]. A urodynamic study by Herrera and Meredith [103] demonstrated day/night differences in bladder capacity and micturition frequency in rats, raising the possibility that the bladder itself can be a direct target for circadian regulation. Although more research is needed to clarify the role of clock genes in urinary functions, recent observations obtained in our own research group support this (Fig. 2). We investigated the daily and circadian patterns of urine volume in wild-type (WT) and per1 -/- per2 -/- (PDK) mice under the light-dark (LD) and constant darkness (dark-dark cycle, DD) conditions. Although both WT and PDK mice exhibited clear daily variations of urine volume in the LD condition, PDK mice lost this rhythmicity just 2 days after being released to DD. Moreover, urinary volume in PDK mice was significantly higher than in WT mice.

Functional clock-dependent rhythms in urinary volume in mice. C57BL/6J male wild-type (WT) and per1-/-per2-/- (PDK) mice were first entrained to a 12 hours:12 hours light-dark photoperiodic cycle in metabolic cages for 2 weeks. Once the animals were fully entrained, urine was collected and volumes were measured every 2 hours over a period of 24 hours (A). Mice were then released to constant darkness (dark-dark cycle, DD). Starting on the second day after lights off, urine was collected and volumes were measured over a circadian cycle (B). Urine volumes measured every 2 hours (left), urine volumes calculated per each phase (middle), and cosinor analysis of urine volumes in WT mice (right) are shown here.
RESEARCH DIRECTIONS
As discussed so far, the circadian control of voiding function is undoubtedly an intriguing possibility and needs the immediate attention of researchers in the field. With several clock mutant animals available, novel approaches are needed to delineate whether urinary functions are under the direct control of the mammalian time-keeping system. First of all, the possible existence of a local bladder clock and its functional significance needs to be addressed. Clock genes oscillating and cycling transcriptome/proteome profiling in the detrusor, urothelium, and sphincter remain unexplored. A second path of research is whether there exist circadian variations in the neural control of the bladder. Because various anatomical locations including the bladder itself, spinal cord, pontine micturition center, and cortical sites contribute to the neural control of bladder function [104-106], possible circadian control in these sites needs to be addressed. Other paths of research include consequences of circadian rhythm disruption in terms of urinary functions and vice versa. Indeed, some research has shown that nocturia and polyuria disrupt sleep architecture and may predict obstructive sleep apnea, whereas acute sleep deprivation results in excess diuresis and natriuresis [107-109]. Also interesting will be the causal relationships between circadian and urological aging [71-74, 110-112].
In modern society, more and more workers are engaged in various shift work and trans-meridian travel, thus repetitively disturbing their circadian rhythms. Moreover, urological problems, such as nocturia, polyuria, and nocturnal enuresis, are the major complaints of the elderly in this aged society. Therefore, the possible circadian control of urinary function is a sure road that needs to be trodden by researchers in the field.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (R11-2008-036-03004-0).
Notes
No potential conflict of interest relevant to this article was reported.