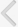|
 |


- Search
| Int Neurourol J > Volume 18(3); 2014 > Article |
|
ABSTRACT
Purpose
Methods
Results
REFERENCES
Fig. 1

Fig. 2
Table 1.
Values are presented as a mean±standard error or number (%). All results are adjusted for age except age variable. P-value was calculated using the general linear model for continuous variables and Cochran-Mantel-Haenszel test for categorical variables. P-value for the trend was determined by the general linear model for continuous variables and Cochran-Mantel-Haenszel test for categorical variables.
Table 2.
| Characteristic |
Sedentary time (hr/day) |
Nonsedentary timea) (hr/day) |
Leisure time (hr/day) |
LTPA (MET-hr/wk) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Low | Medium | High | P-value | P for trend | Low | Medium | High | P-value | P for trend | Low | Medium | High | P-value | P for trend | <22.5 | ≥22.5 | P-value | P for trend | |
| No. | 192 | 204 | 186 | 193 | 195 | 194 | 400 | 178 | 284 | 438 | 144 | ||||||||
| Age (yr) | 64.92±0.67 | 65.00±0.65 | 62.11±0.68 | 0.003 | <0.001 | 65.92±0.66 | 65.09±0.66 | 61.14±0.66 | <0.001 | <0.001 | 65.60±0.57 | 61.00±0.83 | 63.90±0.67 | <0.001 | <0.001 | 64.52±0.45 | 62.63±0.78 | 0.036 | <0.001 |
| Educational status | |||||||||||||||||||
| High school or higher | 71 (27.6) | 90 (35.0) | 96 (37.4) | 0.100 | 0.033 | 95 (37.0) | 80 (31.1) | 82 (31.9) | 0.142 | 0.027 | 97 (37.7) | 57 (22.2) | 103 (40.1) | <0.001 | <0.001 | 178 (69.3) | 79 (30.7) | 0.001 | <0.001 |
| Middle school | 44 (36.7) | 35 (29.2) | 41 (34.2) | 29 (24.2) | 44 (36.7) | 47 (39.2) | 50 (41.7) | 26 (21.7) | 44 (36.7) | 86 (71.7) | 34 (28.3) | ||||||||
| Elementary or below | 77 (37.6) | 79 (38.5) | 49 (23.9) | 69 (33.7) | 71 (34.6) | 65 (31.7) | 117 (57.1) | 42 (20.5) | 46 (22.4) | 174 (84.9) | 31 (15.1) | ||||||||
| Marital status | |||||||||||||||||||
| Married | 181 (33.0) | 190 (34.6) | 178 (32.4) | 0.658 | 0.742 | 182 (33.2) | 180 (32.8) | 187 (34.1) | 0.275 | 0.566 | 247 (45.0) | 119 (21.7) | 183 (33.3) | 0.827 | 0.602 | 411 (74.9) | 138 (25.1) | 0.422 | 0.422 |
| Single | 11 (33.3) | 14 (42.4) | 8 (24.2) | 11 (33.3) | 15 (45.5) | 7 (21.2) | 17 (51.5) | 6 (18.2) | 10 (30.3) | 27 (81.8) | 6 (18.2) | ||||||||
| Smoking status | |||||||||||||||||||
| Never | 48 (30.6) | 52 (33.1) | 57 (36.3) | 0.264 | 0.198 | 46 (29.3) | 51 (32.5) | 60 (38.2) | 0.473 | 0.140 | 58 (36.9) | 42 (26.8) | 57 (36.3) | 0.069 | 0.012 | 119 (75.8) | 38 (24.2) | 0.267 | 0.469 |
| Ex-smoker | 101 (35.0) | 97 (33.6) | 91 (31.5) | 98 (33.9) | 104 (36.0) | 87 (30.1) | 135 (46.7) | 54 (18.7) | 100 (34.6) | 211 (73.0) | 78 (27.0) | ||||||||
| Current smoker | 43 (31.6) | 55 (40.4) | 38 (27.9) | 49 (36.0) | 40 (29.4) | 47 (34.6) | 71 (52.2) | 29 (21.3) | 36 (26.5) | 108 (79.4) | 28 (20.6) | ||||||||
| Drinking status | |||||||||||||||||||
| Never | 34 (35.4) | 30 (31.3) | 32 (33.3) | 0.731 | 0.772 | 32 (33.3) | 30 (31.3) | 34 (35.4) | 0.072 | 0.552 | 40 (41.7) | 27 (28.1) | 29 (30.2) | 0.139 | 0.893 | 75 (78.1) | 21 (21.9) | 0.874 | 0.680 |
| Ex-drinker | 34 (36.2) | 31 (33.0) | 29 (30.9) | 44 (46.8) | 31 (33.0) | 19 (20.2) | 49 (52.1) | 11 (11.7) | 34 (36.2) | 71 (75.5) | 23 (24.5) | ||||||||
| Current drinker | 124 (31.6) | 143 (36.5) | 125 (31.9) | 117 (29.9) | 134 (34.2) | 141 (36.0) | 175 (44.6) | 87 (22.2) | 130 (33.2) | 292 (74.5) | 100 (25.5) | ||||||||
| Body mass index (kg/m2) | |||||||||||||||||||
| <23.0 | 70 (32.7) | 83 (38.8) | 61 (28.5) | 0.645 | 0.864 | 75 (35.1) | 70 (32.7) | 69 (32.2) | 0.261 | 0.187 | 117 (54.7) | 43 (20.1) | 54 (25.2) | 0.002 | <0.001 | 177 (82.7) | 37 (17.3) | 0.009 | 0.003 |
| 23.0-24.9 | 47 (34.1) | 50 (36.2) | 41 (29.7) | 38 (27.5) | 47 (34.1) | 53 (38.4) | 60 (43.5) | 35 (25.4) | 43 (31.2) | 102 (73.9) | 36 (26.1) | ||||||||
| ≥25 | 75 (32.6) | 71 (30.9) | 84 (36.5) | 80 (34.8) | 78 (33.9) | 72 (31.3) | 87 (37.8) | 47 (20.4) | 96 (41.7) | 159 (69.1) | 71 (30.9) | ||||||||
| Chronic diseases | |||||||||||||||||||
| Hypertension | |||||||||||||||||||
| No | 120 (31.8) | 133 (35.3) | 124 (32.9) | 0.705 | 0.435 | 113 (30.0) | 130 (34.5) | 134 (35.5) | 0.079 | 0.037 | 174 (46.2) | 85 (22.6) | 118 (31.3) | 0.443 | 0.251 | 286 (75.9) | 91 (24.1) | 0.542 | 0.542 |
| Yes | 72 (35.1) | 71 (34.6) | 62 (30.2) | 80 (39.0) | 65 (31.7) | 60 (29.3) | 90 (43.9) | 40 (19.5) | 75 (36.6) | 152 (74.2) | 53 (25.9) | ||||||||
| Diabetes | |||||||||||||||||||
| No | 22 (26.8) | 35 (42.7) | 25 (30.5) | 0.256 | 0.554 | 31 (37.8) | 16 (19.5) | 35 (42.7) | 0.011 | 0.549 | 42 (51.2) | 14 (17.1) | 26 (31.7) | 0.514 | 0.464 | 62 (75.6) | 20 (24.4) | 0.987 | 0.987 |
| Yes | 170 (34.0) | 169 (33.8) | 161 (32.2) | 162 (32.4) | 179 (35.8) | 159 (31.8) | 222 (44.4) | 111 (22.2) | 167 (33.4) | 376 (75.2) | 124 (24.8) | ||||||||
| Prostate volume (g) | 29.41±0.72 | 29.86±0.70 | 31.24±0.74 | 0.188 | <0.001 | 30.05±0.73 | 30.46±0.72 | 29.94±0.73 | 0.866 | <0.001 | 29.43±0.62 | 28.93±0.90 | 31.93±0.72 | 0.009 | <0.001 | 29.61±0.48 | 31.78±0.83 | 0.024 | <0.001 |
| <25 | 70 (36.3) | 71 (36.8) | 52 (26.9) | 0.092 | 0.050 | 60 (31.1) | 61 (31.6) | 72 (37.3) | 0.569 | 0.323 | 86 (44.6) | 51 (26.4) | 56 (29.0) | 0.159 | 0.499 | 151 (78.2) | 42 (21.8) | 0.260 | 0.260 |
| ≥25 | 122 (31.4) | 133 (34.2) | 134 (34.5) | 133 (34.2) | 134 (34.5) | 122 (31.4) | 178 (45.8) | 74 (19.0) | 137 (35.2) | 287 (73.8) | 102 (26.2) | ||||||||
| Quality of life score | 2.70±0.07 | 2.65±0.07 | 2.71±0.08 | 0.850 | <0.001 | 2.72±0.07 | 2.65±0.07 | 2.69±0.07 | 0.761 | <0.001 | 2.68±0.06 | 2.71±0.09 | 2.68±0.07 | 0.962 | <0.001 | 2.70±0.05 | 2.64±0.09 | 0.486 | <0.001 |
| Prostate specific antigen (ng/mL) | 1.20±0.06 | 1.12±0.05 | 1.29±0.06 | 0.096 | <0.001 | 1.12±0.06 | 1.25±0.06 | 1.24±0.06 | 0.172 | <0.001 | 1.18±0.78 | 1.16±0.80 | 1.26±0.79 | 0.405 | <0.001 | 1.18±0.04 | 1.26±0.07 | 0.303 | <0.001 |
Values are presented as a mean±standard error or number (%). All results are adjusted for age except age variable. P-value was calculated using the general linear model for continuous variables and Cochran-Mantel-Haenszel test for categorical variables. P-value for the trend was determined by the general linear model for continuous variables and Cochran-Mantel-Haenszel test for categorical variables.
LTPA, leisure time physical activity; MET, metabolic equivalent.