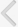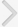Because the kidney filters plasma and excretes the wastes into the urine, urine differs markedly from plasma in osmolality and acidity. The urinary bladder in mammals stores highly permeable molecules such as urea, ammonia, and water to prevent them from crossing the bladder mucosa for prolonged periods without disturbance [1-3]. The mechanism of a permeable barrier has been suggested for these reasons. Sun et al. [3-7] reported that the apical membrane of urinary bladder contains a group of four related transmembrane (TM) proteins, called uroplakins (UPs), that act an exceptional barrier to water, small non-electrolytes, and protons. The asymmetric unit membrane (AUM), which can be seen by electron microscopy, forms the apical plaques of mammalian urothelium [3,8]. The AUM consists of the numerous assembly of four UPs: Ia, Ib, II, and III [4-7]. The UPs are believed to play a role in homeostasis in the urinary bladder mucosa during contraction and relaxation, preventing the AUM from rupturing during bladder distension [9-11].
When this permeability barrier is breached, irritants in the urine reach the bladder submucosa structure, musculature, and nociceptive nerve endings. The inflamed nociceptor fibers can ignite an abnormal sensory circuit and the injured muscular structures lead to urinary frequency, urgency, and painful voiding syndrome [12,13]. Furthermore, long-lasting breaches in the bladder permeability barrier may lead to dysplasia of tumor growth, interstitial cystitis, and non-bacterial chronic cystitis [13,14].
Although extensive studies have been conducted over the past two decades on the pathophysiology of voiding dysfunction, little research has been carried out on the role of UPs in the urinary bladder. This review will summarize the genetic characteristics and significance of UPs in the field of translational research.
GENETIC CHARACTERISTICS OF UROPLAKINS
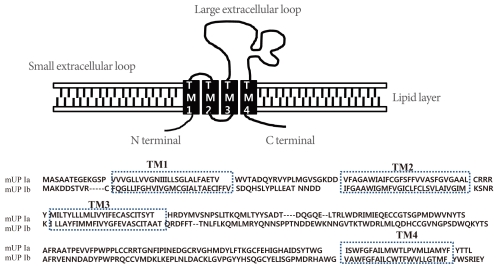
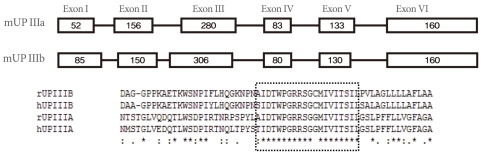
The AUM contains four uroplakins: UP Ia, UP Ib, UP II, and UP III (UP IIIa and UP IIIb) (Fig. 1). The mammalian UPs, including bovine, human, and mouse, are highly conserved [15]. Many tetraspanins such as UP Ia and Ib interact with important single TM domain signaling partner proteins [16,17]. As can be inferred from the name, the tetraspanin superfamily contains four TM domains (Fig. 2) [17]. The tetraspanin superfamily usually contains several conserved amino acid residues in amino acid sequences, which suggests that these UPs may play important roles in cell migration, immunologic signaling, infection, and membrane architecture [18,19]. However, UP II and UP III have only a single TM domain and share a stretch of 12 amino acid residues located on the extracellular side of the single TM domain (RT/SGGMV/IVITV/SL/IL) (Fig. 3) [20]. Of these four major UPs, UP IIIa is the only one that has a relatively large cytoplasmic domain of 50 amino acid residues, which has been postulated to play a role in interacting with the cytoskeleton (Fig. 3) [4,20,21].
The sequence of UP IIIb is related to that of UP IIIa, and they also have six similar exons and share conserved sequences such as IDTWPGRRSC/GMIVITSIL in the extracellular side of the TM domain, which suggests that the conserved area plays an active role for interacting with other important proteins (Fig. 4). UP II is synthesized as a pre (26 amino acids)-pro (59 amino acids)-mature protein (100 amino acids); the mature UP II (15-kDa) can be divided into a long extracellular domain of 71 amino acids and a TM domain of 25 amino acids, with almost no intracellular domain [4]. UP IIIa is synthesized as a pre-protein; the mature protein (47-kDa) contains an apoprotein of 29-kDa plus 18-kDa equivalents of complex glycans [21].
MORPHOLOGY OF UPS
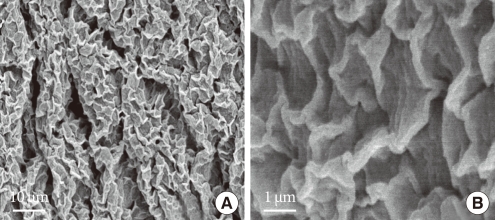
The structure of the urothelial plaque has been studied by using a number of ultrastructural techniques, including atomic force microscopy, quick-freeze deep-etch, and cryo-electron microscopy [8,22,23]. Such data have shown that the apical surface of the mammalian urinary bladder epithelium is covered by numerous scallop-shaped membrane plaques (Fig. 5). The plaque has a unique structure in which its outer leaflet is about twice as thick as the inner one, hence, the name asymmetric unit membrane [24]. The AUM contains four different UPs and integral membrane proteins that assemble into hexagonal plaque particles [20].
Within each UP pair (UP Ia/II and UP Ib/IIIa), there are a total of five TM domains (four TMs for UP Ia and UP Ib, and one TM for UP II and UP III) [25,26]. The five TM helices of each heterodimer are tightly packed, forming six inner-domains and six outer-domains. Furthermore, UPs stabilize a particularly rigid set of lipids within the outer leaflet in the AUM [22].
The AUM structure is also present in fusiform-shaped cytoplasmic vesicles representing a pre-apical membrane compartment. These fusiform-shaped cytoplasmic vesicles create a concave or scallop-shaped plaque in the apical membrane or in terminally differentiated urothelial (umbrella) cells [11,20,27].
FUNCTIONS OF UPS
The amino acid sequences of mammalian UPs are highly conserved, which suggests that they play an essential function in urinary bladder epithelium [1,2,15,16]. This highly specialized membrane is believed to serve as an exceptionally effective permeability barrier [1-3], as a mechanical anchorage site mediating binding of the cytoskeleton to the apical membrane surface [8,5], and as a mechanism for adjusting the apical surface area through the reversible insertion and retrieval of the apical plaques [10,11,27,28]. Furthermore, urothelial plaques are biochemically unique. These membranes are unusually stable in that they remain insoluble in a number of harsh conditions including 2% NP-40, 2% sodium sarcosine, 25 mM NaOH, 9 M urea, and 6 M guanidiumchloride [2].
In normal urothelium, differentiated superficial cells gradually develop from basal and intermediate cells. Because urothelial plaques and their protein subunits, i.e., the UPs, are synthesized in large quantities only by terminally differentiated urothelial cells, the UPs are regarded as a major urothelial differentiation marker [29]. In addition, recent studies indicate that UP Ia may serve as the urothelial receptor for type 1-fimbriated Escherichia coli, which causes 90% of urinary tract infections [30]. UP Ia is only expressed in mouse ventral prostate. This suggests that the mouse ventral prostate may be an adequate locus for acute or chronic bacterial prostatitis study (Fig. 6) [31].
The presence of UP Ib in the ocular surface and respiratory epithelia suggests that UP Ib may play a unique biological role in these tissues (Table 1).
TRAFFICKING
The mechanisms underlying the dynamic changes in the apical membrane compartment are unclear. Discoid vesicles may provide the bladder with a large membrane reserve to adapt to changes in urine volume and to immediately substitute leaky membrane areas. Fusion of the discoid vesicles with the apical plasma membrane results in an increase in the surface membrane area, which might be useful during filling of the bladder when the urothelium has to stretch [11,20,32,33].
The UPs undergo an assembly process that begins in the endoplasmic reticulum (ER) where they form specific heterodimers [20,25,26,34]. Recent studies indicate that UP Ia and UP Ib bind to UP II and UP IIIa, respectively, forming the heterodimers Ia/II and Ib/IIIa before they can exit from the ER [16,26,34]. UP Ib is exceptional because it can exit, by itself, from the ER [26].
Severs and Hicks [35] have shown that, in normal urothelium, 16-nm particles can be detected in early vesicles that have just budded off the Golgi apparatus, which suggests that the two UP heterodimers (Ia/II and Ib/III) interact before they leave the Golgi apparatus to form the 16-nm particle. These data suggest that UPs first form heterodimers in the ER [26], and possibly heterotetramers; the heterotetramers then assemble into the 16-nm particles in the Golgi apparatus or the trans-Golgi network. Then, these particles, once reaching a sufficient density in the post-Golgi vesicles, aggregate to form small, and later, large 2D crystals. In a post-Golgi compartment they form increasingly larger crystalline arrays [8,28], which ultimately cover almost the entire surface of the mature fusiform vesicles. These organelles migrate toward the apical cell periphery where they can fuse with the apical plasma membrane [10,32,33].
Although UP Ia is transported to the plasma membrane only after heterodimerization with UPII, UP Ib is unique in that it can exit from the ER and move to the plasma membrane even when expressed by itself at the surface of urothelial cells of UP IIIa-knockout (KO) mice [26]. On the other hand, in UP II-KO mice, UP Ia remains trapped in the ER [9].
UP EXPRESSION IN CELL LINES
Cultured urothelial cells can synthesize the UPs, but the UPs do not assemble into two-dimensional crystals [36]. Thus, cultured bovine urothelial cells still synthesize large amounts of the UPs, although they almost completely lack the cytoplasmic (fusiform) vesicles that are prominent cellular structures in mature umbrella cells in vivo and which are presumably involved in delivering the UPs to the apical surface [36,37]. Moreover, the apical surface of these cultured urothelial cells completely lack the two-dimensional crystals of 16 nm uroplakin particles [20,36,37]. The inability of regenerating urothelial cells to assemble plaques could be functionally important, because a cell surface laden with the rigid-looking plaques may be incompatible with cell migration and proliferation during wound repair [20]. The RT4 cell line, derived from a grade 1 transitional cell carcinoma (TCC), can form a well differentiated stratified urothelium when seeded onto a stroma. This suggests that the RT4 cell line will be invaluable as a model for urothelial cytodifferentiation and for studying the differential regulation and expression of the UP genes. The expression of the UP Ia and UP II genes remains highly differentiation restricted in superficial TCC. The genes are expressed by the luminal cells of well-differentiated papillae, but the expression is lost from histologically less differentiated areas (Table 2) [38].
KNOCKOUT MICE
KO mice are genetically engineered mice in which a target gene or genes have been turned off through a targeted mutational method. For this reason, the UP KO mice are important animal models for studying the roles of UP genes. With KO mice of UP II or III, the incorporation of fully assembled UP proteins into the apical surface through fusion of the fusiform vesicles with the apical membrane was shown to play a role in the maintenance of the urothelial barriers [9,39].
In UP II KO mice, protein levels of UP Ia, UP Ib, and UP III are reduced 10-20-fold compared with those of the normal control mice. However, the mRNA levels of UP Ia and UP III are up-regulated twofold, whereas that of UP Ib is up-regulated by 10-20-fold in a compensatory manner [9]. In addition, the UP-delivering fusiform vesicles are totally replaced by numerous small, spherical vesicles [9]. In UP II-deficient urothelium, although the UP Ib/III pair can be delivered to the apical surface and the small vesicles (150-200 nm in diameter) accumulate in the UP II-deficient superficial cells, the UP Ib/ III pair by itself cannot form the 16-nm particle, let alone the urothelial plaques. This leads to the entrapment of UP Ia in the ER, but allows the remaining UP Ib/III pair to reach the apical urothelial surface [9]. No 16-nm particle or apical plaques are formed. Some breeding pairs of UP II-deficient mice reproducibly die 8 to 10 days postnatally as a result of renal failure. It seems that major UP defects may cause urinary tract anomalies that, in severe cases, could lead to death. In addition, the levels of many urinary components, including uric acid, creatinine, potassium, sodium, and chloride ions, were slightly reduced, possibly because of defects in mechanisms of urine concentration in UP II KO mice. The concentration of blood urea nitrogen also almost doubled, suggesting a compromised renal function [9].
These results highlight the critical functional importance of UPs in the formation of a specialized urothelial apical surface. Serial sectioning of the urinary tracts from surviving UP II knockout mice reveal areas of the ureter with epithelial polyps or complete epithelial occlusion, which causes structural obstruction [9]. In addition, increased bladder capacity, micturition pressure, and demonstrable nonvoiding contractions are observed in male UP II KO mice compared with normal mice [40].
The detergent-insoluble membrane fraction of the UP III-deficient mouse urothelium shows a significantly reduced number of UPs, whereas that of the UP II-deficient urothelium contains almost no detectable UPs. Together, these results indicate that UP II ablation completely abolishes plaque formation. Because both heterodimers (Ia/II and Ib/IIIa) are required for normal plaque formation, it has been postulated that UP IIIb replaces the necessary function of the UP IIIa to form the Ib/IIIb heterodimer, allowing for the small crystal plaques to develop. Again, knockout of UP II, which does not have a known isoform, leads to the complete absence of plaque formation, supporting the idea that the two UP heterodimers are both critical for plaque formation [9].
In the ablation of UP III, abnormal synthesis and processing of UP Ib, i.e., the level of UP Ib mRNA is greatly increased, whereas the amount of UP Ib protein is reduced [39]. These mice also have a reduced urothelial plaque size, compromised urothelial permeability barrier function, and retrograde flow of urine from the bladder into the ureters (vesicoureteral reflux), resulting in hydronephrosis [3,39]. These results establish that UP III is an integral subunit of urothelial plaques, which contribute to the permeability barrier function of the urothelial apical surface, and suggest that urothelial defects may play a role in vesicoureteral reflux (VUR) [3,39]. The inactivation of the mouse UP III gene results in VUR and hydronephrosis raises the possibility that UP defects may cause VUR in humans [39,41].
The spontaneous bladder contraction activity value for all male and female UP KO mice is significantly greater than that observed in the wild-type mice [40]. Furthermore, the UP II KO mice display significant elevations in spontaneous activity, intermicturition pressure, and micturition pressure relative to both the wild-type and UP IIIa KO mice [40]. The UP IIIa KO mice, in turn, have lower basal pressure and threshold pressure values than do either the UP II or wild-type mice [40]. Cystometric records from both UP II and UP IIIa KO mice (both males and females) reveal demonstrable nonvoiding contractions (i.e., detrusor overactivity ) [40]. Interestingly, the observed detrusor overactivity is more pronounced in the UP II KO mice than in the UP IIIa KO mice [40].
CHANGES IN UP EXPRESSION IN THE URINARY BLADDER AFTER CYCLOPHOSPHAMIDE TREATMENT
The turn-over and differentiation of urothelial cells in the normal urothelium is very slow, and their life span is very long making it difficult to study the detailed sequence of differentiation stages in normal urothelium [42]. However, the turn-over or repair process in the urothelium after chemical insult is very rapid. This dynamic process can help us to understand the pathophysiological mechanisms of the UPs in the urinary bladder. In addition, because chemicals such as cyclophosphamide are well known to induce interstitial cystitis and hemorrhagic cystitis in animal models, it is a highly reasonable approach to determine the role of UPs in the urothelium by applying the cyclophosphamide-induced animal model.
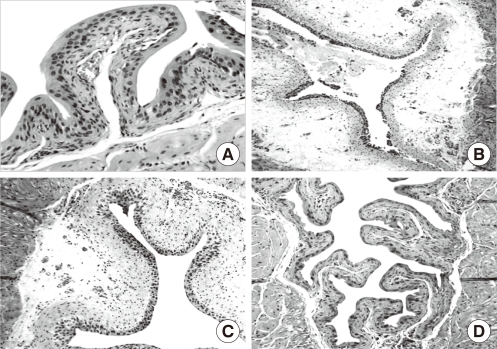
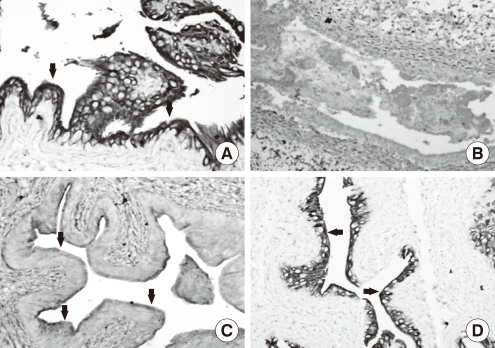
Cyclophosphamide causes mucosal ulceration, transmural edema and epithelial necrosis that are associated with acute hemorrhage (Fig. 7) [43,44]. At 24 hours after cyclophosphamide exposure, the superficial epithelium is detached and intermediate cells are exposed (Fig. 8). The regeneration process is started immediately and is completed within 2 weeks after cyclophosphamide injection [27]. Various stages of urothelial differentiation accompany the regeneration. This is reflected in the successive appearance of microvilli and ropy ridges on the apical surface of the superficial cells [27]. The level of all UP mRNAs, the protein expression of UP II and IIIa by immunoblotting study, and the expression of UP IIIa by immunolocalization study are maximally suppressed within 12 hours; this partially recovers at 24 hours and completely recovers at 72 hours following cyclophosphamide injection in the mouse bladder. Even though a large area of urothelium is lost after cyclophosphamide injection, some focal areas of intact uroepithelium show a significant reduction of UP III expression on immunohistochemical staining. It seems that cyclophosphamide may actively depress the expression of UPs in uroepithelium when they are damaged by cyclophosphamide (Fig. 9) [43]. These results suggest that rapid resealing of injuries to the bladder permeability barrier is of major physiological importance for restoring the protection ability of the bladder.
Vigorous diuresis and agents that can detoxify cyclophosphamide, such as 2-mercaptoethene sulfate (MESNA), have been used to decrease urotoxicity [44]. However, MESNA-treated rats reveal transient and early reductions in the mRNA levels of all UPs [44]. That finding suggests that MESNA treatment may partially preserve the UP expression of mRNA and protein in cyclophosphamide-induced urinary bladder epithelium. In addition, the responses in the level of UPs against cyclophosphamide insult are heterogeneous (i.e., markedly suppressed in UP II and lesser destructive in UP III) [44]. These findings can explain the fact that the bladder protection is not always achieved even after the prophylactic use of MESNA.
In conclusion, significant progress has been made in the characterizations of UPs in genitourinary tract diseases. As a result, it is generally accepted that UPs are actively involved in creating the blood-urine barrier and in urothelial differentiation, trafficking, and genitourinary development in animal models. Lessons from this basic research can lead to an understanding of the clinical significance of the UPs. Urothelial damage and vesical dysfunction in humans can be caused by interstitial cystitis, bladder outlet obstruction, and overactive bladder, as well as vesicoureteral reflux and kidney development. Studies of UPs expression and major UP genetic mutations can explain or help in our understanding of the mechanisms of the above lower urinary tract diseases. In the near future, some pharmacotherapies involving UPs may lead to large clinical benefits in the treatment of genito-urinary diseases.