 |
 |


- Search
| Int Neurourol J > Volume 28(2); 2024 > Article |
|
ABSTRACT
Purpose
Through their biological clocks, organisms on this rotating planet can coordinate physiological processes according to the time of the day. However, the prevalence of circadian rhythm disorders has increased in modern society with the growing number of shift workers, elevating the risk of various diseases. In this study, we employed a mouse model to investigate the effects of urinary rhythm disturbances resulting from dietary changes commonly experienced by night shift workers.
Methods
We established 3 groups based on feeding time and the use of restricted feeding: ad libitum, daytime, and early nighttime feeding. We then examined the urinary rhythm in each group. In addition to the bladder rhythm, we investigated changes in mRNA patterns within the tissues constituting the bladder. Additionally, we assessed the urination rhythm in Per1 and Per2 double-knockout mice and evaluated whether the injection of antioxidants modified the impact of mealtime shift on urination rhythm in wild-type mice.
Results
Our study revealed that a shift in mealtime significantly impacted the circadian patterns of water intake and urinary excretion. In Per2::Luc knock-in mouse bladders cultured ex vivo, this shift increased the amplitude of Per2 oscillation and delayed its acrophases by several hours. Daily supplementation with antioxidants did not influence the mealtime shift-induced changes in circadian patterns of water intake and urinary excretion, nor did it affect the modified Per2 oscillation patterns in the cultured bladder. However, in aged mice, antioxidants partially restored the urinary rhythm.
- This study explored how mealtime shifts, similar to night shift work, affect urinary rhythms in mice. Altered feeding times disrupted circadian patterns of water intake and urination and changed the bladder’s molecular clock. Antioxidants did not mitigate these effects in younger mice but partially restored urinary rhythms in older mice.
As Earth orbits the sun, it experiences a cycle of day and night over approximately 24 hours. Organisms have adapted to this cycle by developing circadian rhythms, which are internal body rhythms that align with Earth’s rotation. In humans, the suprachiasmatic nucleus —considered the master clock —coordinates the individual clocks in each peripheral organ. This nucleus is located in the lower part of the hypothalamus, just above the pituitary gland, and ensures that each organ’s clock is synchronized to the 24-hour cycle [1-3].
Representative genes involved in the circadian rhythm include Per1/2, Bmal1, Rev-erbα, and Cry. These genes are integral components of the clock gene network, which regulates an organism’s adaptability to external stimuli and its physiological processes within the 24-hour cycle. This underscores the vital role of these genes in orchestrating circadian rhythms [4].
The bladder clock is influenced by the time of day, interacting with other organs accordingly. At night, the brain’s arousal level decreases to facilitate rest and sleep, causing the kidneys—the primary excretory organs—to produce a reduced volume of urine. Simultaneously, the bladder’s capacity to store urine increases. During the daytime, opposite processes are observed [5]. Among the various organs involved in urination, the present study was focused on the bladder.
Shifts involving forced asynchrony between working hours and environmental light-dark (LD) cycles have become increasingly prevalent in modern society. Shift workers must align their sleep-wake cycles and food intake with their imposed work schedules [6, 7]. This can alter hormone secretion and physiological rhythms, including temperature regulation. Such disruptions in circadian rhythms can have pathological consequences. Indeed, shift work is associated with so-called shift work disorders, which include metabolic disorders, gastrointestinal symptoms, sleep disturbances, and psychiatric disorders [8-10].
The experiment utilized a shift work model in mice, altering their feeding times to reflect the irregular schedules inherent to contemporary shift work. Typically, nocturnal mice consume most of their food in the period after nightfall [11]. However, shifting the normal feeding time by 6 hours, either earlier or later, can disturb both metabolic rhythms and behavior in young adult male mice [12]. Given the key role of the feeding-fasting cycle in metabolism, time-restricted feeding serves as an effective proxy for the conditions experienced by shift workers and the consequent metabolic impacts [13].
Building on research demonstrating increased oxidative stress and subsequent insulin resistance in mice due to shifts in mealtime [14], our study examined the effects of mealtime-induced oxidative stress on bladder rhythm. We specifically analyzed changes in urination patterns following these shifts. Furthermore, we investigated the effects of potent antioxidants, namely melatonin and cyanidin-3-glucoside (C3G), on blood levels of reactive oxygen species and antioxidative capacity. This research was performed to clarify the role of oxidative stress in bladder function disruption when mealtimes are altered, as well as to evaluate the potential therapeutic advantages of antioxidants in mitigating these effects.
In this study, we examined the impact of meal timing on the bladder’s circadian rhythm to understand potential urinary system disorders common among shift workers. We also assessed the potential therapeutic effects of antioxidants in alleviating these disturbances. Our findings contribute to the understanding of the relationship between circadian disruption and urinary health, offering insights into preventive measures and therapeutic interventions for individuals with irregular meal patterns, especially those engaged in shift work.
C57BL/6J mice were purchased from DBL (Seoul, Korea) and acclimated to an ambient temperature of 23°C ±1°C with a 12:12 LD photoperiodic cycle. The animals were individually housed in clean, lightproof animal rack cabinets (Shin Biotech, Seoul, Korea) with continuous air ventilation. During the light phase, light intensity at the bottom of the cage was maintained at 350–450 lux. The mice had continuous access to standard chow (Purina chow food: 20% crude protein, 4.5% crude fat, 6% crude fiber, 7% crude ash, 0.5% calcium, and 1% phosphorus) and water until the initiation of time-restricted feeding.
Fig. 1A illustrates the experimental timeline for the restrictive feeding schedule. Mice were randomly divided into 3 groups. The ad libitum (AF) group had continuous access to food throughout the duration of the experiment. The daytime feeding (DF) group was permitted to eat for a 5-hour period daily, starting 6 hours earlier than the typical feeding time of mice. Finally, the early nighttime feeding (ENF) group was allowed to eat for 5 hours during their usual feeding time each day. The ENF group was established to evaluate the effects of a 5-hour restricted feeding schedule in comparison to the AF group. The restrictive feeding protocol was conducted for 4 weeks, after which it was maintained concurrently with additional experiments.
Fig. 1B outlines the experimental timeline for antioxidant administration. The mice were randomly divided into 4 groups: an AF group and 3 groups subjected to restricted feeding on the DF schedule. The groups on the DF regimen received daily intraperitoneal injections of either vehicle, melatonin (Sigma-Aldrich, St. Louis, MO, USA), or C3G (JBK School of Medicine, Seoul, Korea). Both melatonin and C3G are antioxidants. These compounds were initially dissolved in absolute ethanol and subsequently diluted 50-fold with saline solution immediately before administration. The dosage for both melatonin and C3G was 10 mg/kg body weight. Restrictive feeding was implemented for the first 4 weeks, with antioxidant injections commencing in week 5. The experiments continued for an additional 2 weeks, during which restrictive feeding and antioxidant treatments were administered concurrently.
Wild-type (WT) mice were individually housed in metabolic cages (Jeungdo, Seoul, Korea) to assess water consumption, food intake, and urine excretion in response to mealtime shift, daily melatonin supplementation, or a combination of both. On day 8, water consumption, food intake, and urine excretion were measured at 2-hour intervals over a 24-hour period. On day 10, the lights were turned off. Two days after that, the same variables were measured every 2 hours under constant dark conditions throughout the circadian cycle. These experiments were conducted in duplicate to establish the weekly pattern, and the results were graphed as average values.
Adult Per2::Luc knock-in mice were sacrificed by cervical dislocation, and whole bladder samples were promptly collected. These samples were utilized for real-time monitoring of Per2 promoter activity, with the specific approach depending on the experiment used. For each whole bladder sample, the outlet was secured with a thread to prevent leakage, and any residual urine was aspirated using a syringe. The bladder was filled with modified Eagle medium (Gibco, Carlsbad, CA, USA) supplemented with 25% fetal bovine serum, 1% penicillin-streptomycin, 36mM glucose, and 0.3mM luciferin (Promega, Madison, WI, USA). The samples were fixed in a 35-mm culture dish (Corning, Tewksbury, MA, USA) using cell strainers (SPL, Seoul, Korea). Subsequently, bioluminescence was recorded over a period of 6 days using a Kronos device (ATTO, Tokyo, Japan).
Total RNA was extracted from the bladder, including the detrusor smooth muscle, uroepithelial layer, and sphincter smooth muscle, using the single-step acid guanidinium thiocyanate-phenol-chloroform extraction method [13]. The RNA concentration was measured with an ND-100 spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). For reverse transcription, each RNA sample was diluted to 1 μg/10 μL and incubated with 200 ng of random hexamers at 65°C for 5 minutes, followed by rapid cooling on ice for 2 minutes. To each sample, we added 4 μL of 5X reverse transcriptase buffer, 4 μL of 2.5mM deoxyribonucleotide triphosphates, 0.5 μL of RNase inhibitor (40 units, 2310A; Takara Bio Inc., Shiga, Japan), and 0.5 μL of Reverse Transcriptase M-MLV (200 units, 2640A; Takara Bio Inc.). The samples were then incubated at 37°C for 1 hour and at 70°C for 10 minutes. The real-time reverse transcription polymerase chain reaction (RT-PCR) procedure, conducted using the LightCycler device (Roche, Indianapolis, IN, USA), has been previously described [15]. Standards were prepared by pooling complementary DNA sample dilutions in 1mM Tris buffer. RT-PCR was performed using 2X SYBR Premix EX Taq (RR041A, Takara Bio Inc.) in a LightCycler 1.5. The expression level of the TATA box-binding protein was used as a reference to normalize gene expression levels. The primer sequences used for real-time RT-PCR were as follows: Per1 up, 5'-GTGTCGTGATTAAATTAGTCAG-3'; Per1 dn, 5'-ACCACTCATGTCGTCTGGGCC-3'; Rev-erbα up, 5'-AAGACATGACGACCTGGAC-3'; Rev-erbα dn, 5'-GAGTCAGGGACTGGAAGCTG-3'.
Mice were divided into 3 groups to investigate the effects of a mealtime shift on voiding rhythm: the AF group, which had access to food at any time; the DF group, for which eating was restricted to a 5-hour period during the light phase, starting 6 hours earlier than the usual feeding time for mice; and the ENF group, which was limited to a 5-hour feeding window starting after the onset of the dark phase, a time of typical consumption for the animals (Fig. 1A). After 4 weeks of the mealtime shift, water intake and urine volume were measured for each group. Regarding light, this experiment was conducted under both an LD cycle and conditions of constant darkness (DD). Under the LD cycle, the AF mice exhibited low water intake and urine output during the light phase, which is their natural rest period; in contrast, both measurements increased during the dark phase, their active period (Fig. 2A and B). This pattern was also observed under the DD cycle, which enabled observation of the intrinsic rhythm (Fig. 2C and D). However, in both the DF and ENF groups, the water intake rapidly increased upon food availability, regardless of light conditions. Urine output followed suit, rising approximately 6 hours after the increase in water consumption. Furthermore, the patterns observed under both LD and DD cycles were similar for these groups. These findings suggest that the voiding rhythm is significantly influenced by food intake rather than by the day-night cycle.
To investigate the effect of DF on the bladder, we utilized mice with a knock-in (KI) of Per2-luciferase, a representative circadian gene. The bladders were harvested from Per2::KI mice after 4 weeks of restricted feeding. Each bladder was tied from the organ to the ureter with a thread to form a balloon shape and then filled with luciferase medium. Subsequently, the bladder was fixed in a culture dish containing the same medium. We measured the bioluminescence of the bladder tissue using a Kronos device, which enables real-time bioluminescence monitoring (Supplementary Fig. 1). Based on the bioluminescence data obtained after 4 weeks of AF, DF, and ENF, the oscillation of the Per2 gene was more pronounced in the bladders of mice subjected to DF than in the AF group. Conversely, the ENF group, which was anticipated to feed concurrently with the AF group, exhibited a diminished rhythm compared to the AF group (Fig. 3A). A consistent pattern was noted in the free-running period (FRP). One-way analysis of variance revealed a significant difference among the 3 groups (P=0.0012). Specifically, the FRP was significantly longer in the DF group relative to the AF group, while no significant difference was found between the AF and ENF groups (Fig. 3B).
In Per1 and Per2 double-knockout (PDK) mice, water intake and urine volume were measured following 4 weeks of AF, DF, or ENF. These measurements were taken under both LD and DD conditions. In the presence of light, the patterns of water intake and urine volume in all groups resembled those of WT mice, according to the light cycle (Figs. 2A and B, 4A and B). However, in the DD environment, the rhythm of the AF group completely disappeared. In contrast, the DF and ENF knockout groups, which experienced restricted feeding, maintained rhythms of water intake and urine volume that were not significantly different from those observed in WT mice (Figs. 2C and D, 4C and D). These data suggest that in mice lacking clock genes, the urinary rhythm is lost but can be reinstated through dietary regulation. Thus, clock genes are crucial for the regulation of the mouse bladder clock; however, external factors, such as the timing of food intake, appear to have a more pronounced effect on sustaining the peripheral clock of the bladder.
As presented in Fig. 1B, an experiment was conducted on groups of mice that underwent a mealtime shift for 6 weeks and received an antioxidant for 2 weeks. The experiment employed a metabolic cage and was performed in duplicate. In the AF group, water intake and urine output decreased during the daytime rest period and tended to increase at night, when mice are active. These results were consistent, even in DD conditions. The mice exhibited a distinct rhythm of water consumption and urine excretion (Fig. 5A–D). However, in the DF groups under LD cycles, water intake increased rapidly starting at the time of feeding, a pattern which was also observed under DD conditions (Fig. 5A and C). Approximately 6 hours following the rise in water consumption, urine volume increased significantly in all DF groups, with similar trends under both LD and DD conditions (Fig. 5B and C) regardless of antioxidant treatment. Throughout the experiment, no significant differences were found among the groups in terms of food consumption, water intake, or urine output. Therefore, the intrinsic voiding rhythm induced by the mealtime shift was significantly impacted by the feeding time, and this change was not influenced by antioxidant administration.
A mealtime shift was implemented for a 20-week period in 28-week-old mice. Concurrently, antioxidants were administered for the same duration. In the AF mice, we observed a weakening of both water intake and voiding rhythm over time. In contrast, the DF group retained a voiding rhythm. Notably, this rhythm was more robustly maintained in the group that received C3G (Fig. 6A and B). The same effect was achievable in a DD setting (Fig. 6C and D). Consequently, the voiding rhythm seems to become weaker with age, but this change can be mitigated by the administration of antioxidants like C3G.
The impact of altered meal timing on bladder rhythm in young adult mice was examined. Notably, DF, which simulates shift work conditions, strongly influenced bladder rhythm in these adult mice.
The recently studied concept of intermittent fasting differs substantially from the mealtime shift model employed in this study. Research has demonstrated that intermittent fasting, which involves enduring extended periods of hunger and consuming food within a restricted timeframe during active phases, is beneficial for managing metabolic syndromes, including obesity and diabetes [16, 17]. Humans have evolved to eat during periods of activity and to fast during periods of rest. In contrast, the mealtime shift model disrupts the circadian rhythm by promoting food intake during the body’s rest period.
In contrast to mice, which are nocturnal creatures that generally feed at night and rest during the day, the opposite patterns of rest and activity are evident in humans when not disrupted, such as by shift work. This study sought to replicate shift work conditions by subjecting mice to a reversed LD schedule, compelling them to be active and feed during their typical rest phase [18]. Throughout the experiment, food was made available during the light hours, a time when mice would normally be resting, thus impeding their ability to achieve complete rest. The design of this experiment was based on a model that our group described previously in published work [12, 14].
It is well-established that mice consume most of their daily food intake within 6 hours after nightfall [11, 12]. To investigate the impact of time-restricted feeding, we compared the ENF group, for which food availability was limited to a 6-hour window after nightfall, to the AF group, which was given unrestricted access to food throughout the day and night. This approach enabled us to discern any differences attributable to time-restricted feeding as opposed to free feeding conditions.
Disrupting the natural 24-hour rhythm, such as with shift work, can increase the risk of various metabolic and chronic disorders [8, 9, 19]. Shift workers are subject to a range of abnormal conditions, including altered exposure to light and changes in sleep and mealtime patterns. While changes in mealtime alone do not fully replicate the effects of shift work, they can significantly disrupt circadian rhythms [12]. Disturbances in circadian rhythm have been linked to the development of several conditions, including nocturia, which is characterized by the need to wake up at night to urinate. Previously considered a normal part of aging, nocturia is now recognized as a disorder caused by disruptions in circadian rhythm. Despite its prevalence, the evaluation and treatment of nocturia are often inadequate [20]. Therefore, it is imperative to investigate the dysregulation of urinary function related to circadian disturbances and to identify new therapeutic targets.
Several previous studies have demonstrated the existence of a clock in the bladder [21]. We designed an experiment to determine how a shift in mealtime affects this organ (Fig. 1A). To assess the urination rhythm, we conducted tests under both LD and DD cycles. The LD cycle was used to observe the rhythm under conditions with light cues, simulating a typical environmental setting. In contrast, the DD condition was employed to investigate the urination rhythm in the absence of cues such as light, thereby confirming the inherent, endogenous rhythm independent of external factors. We found that the bladder’s urinary rhythm was controlled by its own clock under both LD and DD conditions, and water intake increased when feeding was restricted. After approximately 5 hours, urine volume then increased (Fig. 2A–D). To investigate the impact of restricted feeding on bladder rhythm, we measured bioluminescence in real time using Per2::KI mice. Similar to the urination rhythm, the bladder rhythm was further strengthened by DF (Fig. 3A). Compared to AF mice, the FRP in these animals exhibited a longer cycle (Fig. 3B). Further studies are needed to elucidate the relationship between the bladder and voiding rhythm as the cycle lengthens. DF was confirmed to significantly affect both the urinary rhythm and the bladder clock. Subsequently, we examined the mRNA levels of clock genes in bladder tissue to identify any differences between AF and DF (Supplementary Fig. 2). In the AF group, Per1 and Rev-erbα (the primary clock genes) exhibited rhythms in all 3 layers of the bladder with a cycle of 1 day. However, in the DF group, which was restricted to a daily 5-hour feeding window, the rhythm either disappeared or became very weak throughout the experiment. This suggests that food intake alters the urinary rhythm at the genetic level. PDK mice, in which both Per1 and Per2 are knocked out, typically exhibited no urinary rhythm; however, the imposition of restricted feeding induced a rhythm similar to that of WT mice (Figs. 2D, 4D).
Previous research has demonstrated that advancing mealtimes by 6 hours for a 4-week period in mice significantly improves antioxidant capacity during the early night, concomitant with increased levels of reactive oxygen species [14]. These changes lead to an imbalance between oxidative stress and antioxidant activity, indicating a potential increase in oxidative damage during the light phase. Given the link between oxidative stress and urinary disorders documented in prior studies [22-24], our investigation was conducted with the hypothesis that changes in urinary rhythm in response to meal timing might be linked to oxidative stress. Accordingly, the antioxidants melatonin and C3G were administered at zeitgeber time 23, just before the anticipated onset of oxidative damage due to the shift in mealtime. We then examined the changes in urination rhythm in response to the shift in meal timing and the impact of antioxidant administration (Fig. 1B) [14, 25-28]. The altered voiding rhythm under DF was not significantly affected by the administration of antioxidants (Fig. 5A–D). Additionally, through activity monitoring to evaluate the mice’s circadian patterns, we noted food-anticipatory activity in those subjected to restricted feeding (data not shown), while no significant differences were found in the other mice [14]. However, novel findings emerged from an experiment involving 28-week-old mice that were administered DF and antioxidants for 20 weeks. With age, the voiding rhythm in the AF group gradually diminished from an original value of 1 day, while the voiding rhythm present under DF conditions was maintained with age. Notably, the voiding rhythm was most pronounced in the group that received C3G (Fig. 6). Similar outcomes were observed in experiments assessing the rhythm of the bladder itself. The bladder’s circadian rhythm was more robust in the DF group compared to the AF group, and the bladder rhythm was further strengthened in the C3G-administered group (Supplementary Fig. 3). However, these findings are associated with aging and warrant further in-depth studies.
In conclusion, this study demonstrated that a 12-hour shift in mealtime significantly affects urinary rhythm in young adult mice, altering bladder rhythm and changing the mRNA expression patterns of major bladder tissues. These findings suggest a potential link to bladder-related diseases commonly seen in shift workers. However, the relationship between circadian disturbances and bladder-associated diseases requires further investigation. Nocturia, a bladder-related circadian disorder, has an unclear underlying mechanism. While diet and hydration play key roles in the regulation of urination rhythm, this study revealed that these factors also influence mRNA levels. Despite the insights gained, our understanding of nocturia remains incomplete. The results of this study underscore the need for more in-depth research into the connection between circadian disturbances and dysuria.
SUPPLEMENTARY MATERIALS
Supplementary Figs. 1-3 can be found via https://doi.org/10.5213/inj.2448144.072.
Supplementary Fig. 1.
Schematic diagram illustrating the protocol for real-time monitoring of Per2 promoter activity in the bladders of Per2::Luc knock-in mice. Detailed descriptions of the experimental methods can be found in the MATERIALS AND METHODS section.
Supplementary Fig. 2.
Presence of a functional circadian clock in 3 distinct regions of the bladder. The detrusor smooth muscle (A, B), sphincter smooth muscle (C, D), and uroepithelial layer (E, F) of the bladders of adult Per2::Luc mice were dissected and examined regarding Per2 promoter-driven luciferase activity using a dissecting microscope. Clock gene expression was analyzed in the 3 bladder tissues, taken from mice under the AF and DF feeding schedules. Both AF and DF groups were acclimated to a 12:12 LD photoperiodic cycle for 1 week before being kept under DD conditions. Two days after the cessation of the LD cycle, the mice were sacrificed by cervical dislocation at a predetermined circadian time. Levels of Per1 (A, C, and E) and Rev-erbα (B, D, and F) mRNA were quantified using real-time polymerase chain reaction. All mRNA levels were normalized to the Tbp mRNA level and are presented as mean±standard error of the mean (n=4 per group). AF, ad libitum feeding; DF, daytime feeding; LD, light-dark; DD, constant darkness; mRNA, messenger RNA.
Supplementary Fig. 3.
Recordings of bioluminescence from whole bladders of Per2::Luc mice. (A) The bioluminescence activity of Per2 promoter-driven luciferase was assessed in Per2::Luc mice from the AF, DF+V, DF+MEL, and DF+C3G groups. Adult Per2::Luc mouse bladders were dissected and examined for Per2 promoter-driven luciferase activity using a dissecting microscope. Bioluminescence was continuously recorded for over 6 days with a Kronos apparatus, as described in the Materials and Methods section. This figure presents an example of the bioluminescence recordings. (B) The mean bioluminescence levels over 6 days were calculated for each group and compared with the average values. All data are expressed as mean±standard error of the mean (n=6–8 per group). AF, ad libitum feeding; DF, daytime feeding; V, vehicle; MEL, melatonin; C3G, cyanidin-3-glucoside. *P<0.1 vs. the AF group. ***P<0.001 vs. the AF group.
NOTES
Grant/Fund Support
This study received backing from the Basic Research Support Program via the National Research Foundation (NRF) of Korea, sponsored by the Korean government (Grant No. 2015R1D1A1A01060982) in the field of science and engineering.
Research Ethics
All animal experiments were approved by the Institutional Animal Care and Use Committee of Kyung Hee University (permit number: KHUASP(SE)-15-113).
Conflict of Interest
KJC and KHK, members of the Editorial Board of International Neurourology Journal, is the corresponding authors of this article. However, they played no role whatsoever in the editorial evaluation of this article or the decision to publish it. Except for that, no potential conflict of interest relevant to this article was reported.
ACKNOWLEDGEMENTS
The authors wish to express their gratitude to everyone who provided technical expertise and support for this project.
REFERENCES
1. Honma S. Development of the mammalian circadian clock. Eur J Neurosci 2020;51:182-93. PMID: 30589961



2. Rosenwasser AM, Turek FW. Neurobiology of circadian rhythm regulation. Sleep Med Clin 2015;10:403-12. PMID: 26568118


3. Mohawk JA, Green CB, Takahashi JS. Central and peripheral circadian clocks in mammals. Annu Rev Neurosci 2012;35:445-62. PMID: 22483041



4. Rijo-Ferreira F, Takahashi JS. Genomics of circadian rhythms in health and disease. Genome Med 2019;11:82. PMID: 31847894




5. Negoro H, Kanemastsu A, Yoshimura K, Ogawa O. Chronobiology of micturition: putative role of the circadian clock. J Urol 2013;190:843-9. PMID: 23429068


6. Bekkers MB, Koppes LL, Rodenburg W, van Steeg H, Proper KI. Relationship of night and shift work with weight change and lifestyle behaviors. J Occup Environ Med 2015;57:e37-44. PMID: 25749131


7. Balieiro LC, Rossato LT, Waterhouse J, Paim SL, Mota MC, Crispim CA. Nutritional status and eating habits of bus drivers during the day and night. Chronobiol Int 2014;31:1123-9. PMID: 25231504


8. Zimmet P, Alberti KGMM, Stern N, Bilu C, El-Osta A, Einat H, et al. The circadian syndrome: is the metabolic syndrome and much more! J Intern Med 2019;286:181-91. PMID: 31081577




9. Deng N, Kohn TP, Lipshultz LI, Pastuszak AW. The relationship between shift work and men’s health. Sex Med Rev 2018;6:446-56. PMID: 29371140


10. Boivin DB, Boudreau P. Impacts of shift work on sleep and circadian rhythms. Pathol Biol (Paris) 2014;62:292-301. PMID: 25246026


11. Sanchez-Alavez M, Klein I, Brownell SE, Tabarean IV, Davis CN, Conti B, et al. Night eating and obesity in the EP3R-deficient mouse. Proc Natl Acad Sci U S A 2007;104:3009-14. PMID: 17307874



12. Yoon JA, Han DH, Noh JY, Kim MH, Son GH, Kim K, et al. Meal time shift disturbs circadian rhythmicity along with metabolic and behavioral alterations in mice. PLoS One 2012;7:e44053. PMID: 22952870



13. Guerrero-Vargas NN, Espitia-Bautista E, Buijs RM, Escobar C. Shiftwork: is time of eating determining metabolic health? Evidence from animal models. Proc Nutr Soc 2018;77:199-215. PMID: 29307314


14. Park J, Kim J, Yun Y, Han DH, Kim K, Hong J, et al. Daily injection of melatonin inhibits insulin resistance induced by chronic mealtime shift. Physiol Rep 2022;10:e15227. PMID: 35343087




15. Lee YJ, Han DH, Park Y, Cho S. Circadian regulation of low-density lipoprotein receptor promoter activity by CLOCK/BMAL1, Hes1 and Hes6. Exp Mol Med 2012;44:642-52. PMID: 22913986



16. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr 2019;39:291-315. PMID: 31180809



17. Patterson RE, Laughlin GA, Sears DD, LaCroix AZ, Marinac C, Gallo LC, et al. Intermittent fasting and human metabolic health. J Acad Nutr Diet 2015;115:1203-12. PMID: 25857868



18. Lennernäs M, Hambraeus L, Akerstedt T. Shift related dietary intake in day and shift workers. Appetite 1995;25:253-65. PMID: 8746965


19. Hatori M, Panda S. Response of peripheral rhythms to the timing of food intake. Methods Enzymol 2015;552:145-61. PMID: 25707276


20. Boroda JU, De Leon B, Khosla L, Chobufo MD, Rahman SN, Lazar JM, et al. Application of the sleep C.A.L.M. tool for assessing nocturia in a large nationally representative cohort. Int Neurourol J 2024;28(Suppl 1):55-61. PMID: 38461857




21. Noh JY, Han DH, Yoon JA, Kim MH, Kim SE, Ko IG, et al. Circadian rhythms in urinary functions: possible roles of circadian clocks? Int Neurourol J 2011;15:64-73. PMID: 21811695




22. Khosla L, Gong S, Weiss JP, Birder LA. Oxidative stress biomarkers in age-related lower urinary tract disorders: a systematic review. Int Neurourol J 2022;26:3-19. PMID: 35368181




23. de Rijk MM, Wolf-Johnston A, Kullmann AF, Taiclet S, Kanai AJ, Shiva S, et al. Aging-associated changes in oxidative stress negatively impacts the urinary bladder urothelium. Int Neurourol J 2022;26:111-8. PMID: 35793989




24. Andersson KE. Oxidative stress and its relation to lower urinary tract symptoms. Int Neurourol J 2022;26:261-7. PMID: 36599334




25. Yu GM, Tan W. Melatonin inhibits lipopolysaccharide-induced inflammation and oxidative stress in cultured mouse mammary tissue. Mediators Inflamm 2019;2019:8597159. PMID: 30890898




26. Skovsø S. Modeling type 2 diabetes in rats using high fat diet and streptozotocin. J Diabetes Investig 2014;5:349-58. PMID: 25411593




27. Li G, Hou G, Lu W, Kang J. Melatonin protects mice with intermittent hypoxia from oxidative stress-induced pancreatic injury. Sleep Biol Rhythms 2011;9:78-85. 
28. Ke Z, Liu Y, Wang X, Fan Z, Chen G, Xu M, et al. Cyanidin-3-glucoside ameliorates ethanol neurotoxicity in the developing brain. J Neurosci Res 2011;89:1676-84. PMID: 21671257



Fig. 1.
Experimental scheme. (A) Young adult C57BL/6J male mice (8 weeks old) were initially entrained to a 12:12 LD cycle for 1 week with ad libitum access to food and water. The mice were then randomly divided into 3 groups: AF, DF (ZT6–ZT11), and ENF (ZT12– ZT17). Water was consistently available for all groups. The mice were individually housed in metabolic cages and subjected to their respective feeding regimens for 4 consecutive weeks. (B) Young adult C57BL/6J male mice (8 weeks old) were initially entrained to a 12:12 LD cycle for 1 week with ad libitum access to food and water. The mice were then randomly divided into 4 groups: AF, DF (ZT6– ZT11) with a daily i.p. injection of vehicle (2% ethanol in physiological saline) at ZT23 (DF+V), DF (ZT6–ZT11) with a daily i.p. injection of MEL (10 mg/kg body weight) at ZT23 (DF+MEL), and DF (ZT6–ZT11) with a daily i.p. injection of C3G (10 mg/kg body weight) at ZT23 (DF+C3G). The mice were fed either ad libitum or in a time-restricted manner for 6 consecutive weeks. Vehicle, MEL, and C3G were administered over the last 2 weeks. Drug administration was performed under dim red light. Water was continuously accessible for all groups, and the mice were individually housed in metabolic cages according to their feeding regimen, as depicted in the schematic. LD, light-dark; AF, ad libitum feeding; DF, daytime feeding; ZT, zeitgeber time; i.p., intraperitoneal; MEL, melatonin; V, vehicle; C3G, cyanidin-3-glucoside.

Fig. 2.
Daily circadian patterns of water consumption and urine excretion under daytime feeding for 4 weeks. The experiment was conducted according to the scheme in Fig. 1A. On the eighth day, water intake (A) and urine volume (B) were measured at 2-hour intervals throughout the day. The data for the 12-hour day, 12-hour night, and full 24-hour periods were pooled and presented graphically. On day 10, the lights were turned off to assess the internal metabolic rhythm. Two days after that, water intake (C) and urine volume (D) were measured under DD conditions at 2-hour intervals. These experiments were conducted in duplicate to establish the weekly pattern, and the results are displayed on the graph as average values. All data are expressed as mean±standard error of the mean (n=6 per group). LD, light-dark; AF, ad libitum feeding; DF, daytime feeding; ZT, zeitgeber time; ENF, early nighttime feeding; DD, constant darkness.

Fig. 3.
Recordings of bioluminescence from whole bladders of Per2::Luc mice. (A) Bladders from Per2::Luc mice in the AF, DF, and ENF groups were assessed for Per2 promoter-driven luciferase activity. Adult Per2::Luc mouse bladders were dissected and examined for this activity using a dissecting microscope. Bioluminescence was continuously recorded for over 6 days with a Kronos apparatus, as described in the Methods section. This figure presents an example of the bioluminescence recordings. (B) The FRP was determined by averaging the peak-to-peak time from the bioluminescence recordings for each group. All data are expressed as mean±standard error of the mean (n=6–8 per group). AF, ad libitum feeding; DF, daytime feeding; ENF, early nighttime feeding; FRP, free-running period. **P<0.01 vs. the AF group. ##P<0.01 vs. the DF group.

Fig. 4.
Daily and circadian patterns of water consumption and urine excretion in PDK mice under daytime feeding for 4 weeks. Young adult male PDK mice were entrained to a 12:12 LD photoperiodic cycle and then divided into 3 groups, AF, DF, and ENF, for 4 consecutive weeks. The mice were individually housed in metabolic cages with time-restricted feeding regimens. On the eighth day, water intake (A) and urine volume (B) were measured at 2-hour intervals throughout the day. The data for the 12-hour day, 12-hour night, and full 24-hour periods were pooled and presented graphically. On day 10, the lights were turned off to assess the internal metabolic rhythm. Two days after that, water intake (C) and urine volume (D) were measured under DD conditions at 2-hour intervals. These experiments were conducted in duplicate to establish the weekly pattern, and the results are displayed on the graph as average values. All data are expressed as mean±standard error of the mean (n=6 per group). PDK, Per1 and Per2 double-knockout; LD, light-dark; AF, ad libitum feeding; DF, daytime feeding; ENF, early nighttime feeding; DD, constant darkness.

Fig. 5.
Daily circadian patterns of water consumption and urine excretion under daytime feeding for 6 weeks. The experiment was conducted according to the scheme in Fig. 1B. On the eighth day, water intake (A) and urine volume (B) were measured at 2-hour intervals over a 24-hour period. The data for the 12-hour day, 12-hour night, and full 24-hour periods were pooled and presented graphically. On day 10, the lights were turned off to assess the internal metabolic rhythm. Two days after that, water intake (C) and urine volume (D) were measured under DD conditions at 2-hour intervals. These experiments were conducted in duplicate to establish the weekly pattern, and the results are displayed on the graph as average values. All data are expressed as mean±standard error of the mean (n=6–8 per group). LD, light-dark; AF, ad libitum feeding; DF, daytime feeding; MEL, melatonin; V, vehicle; C3G, cyanidin- 3-glucoside; DD, constant darkness.
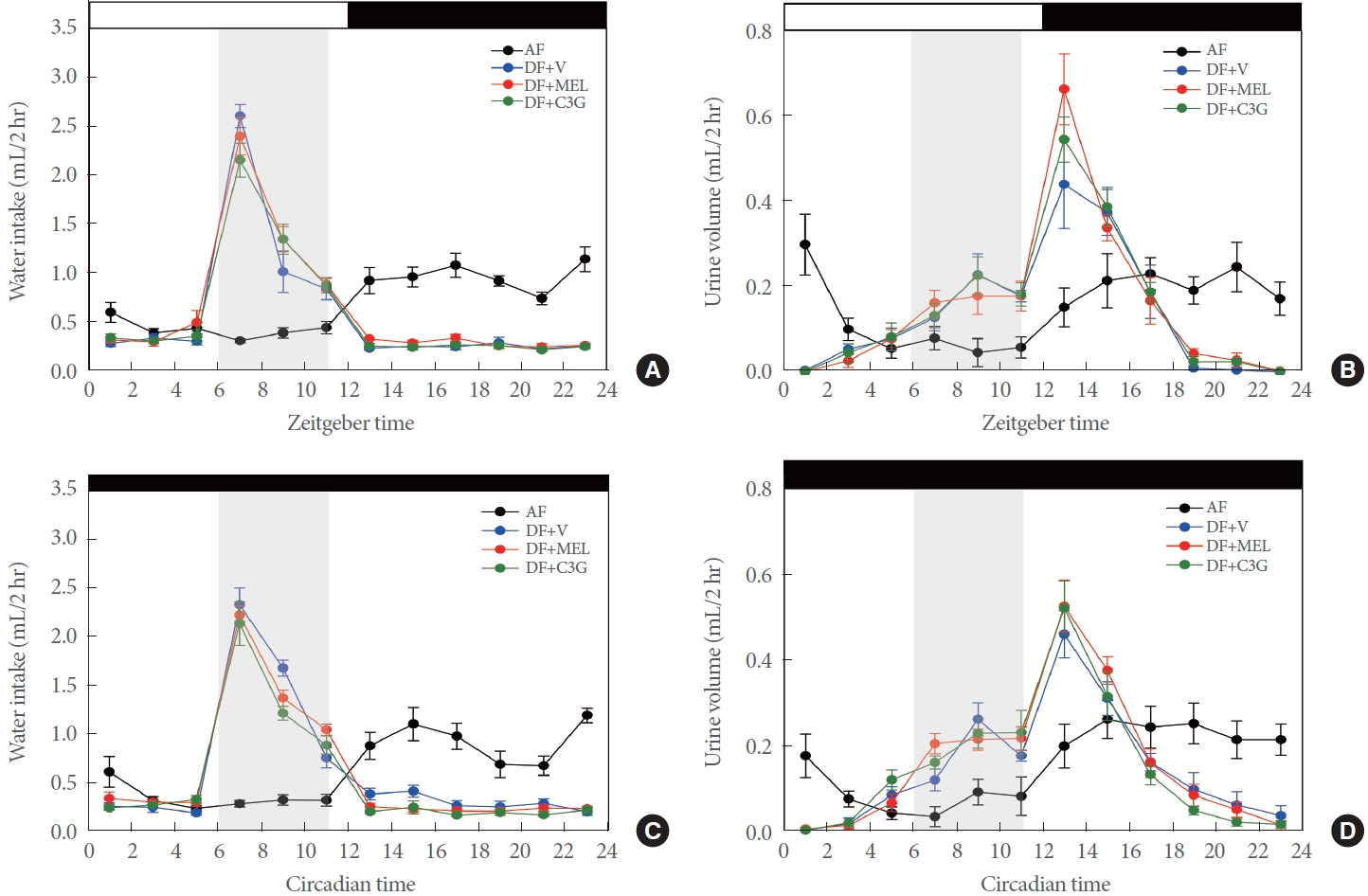
Fig. 6.
Daily and circadian patterns of water consumption and urine excretion under daytime feeding for 17–20 weeks. Young adult male mice were initially entrained to a 12:12 LD photoperiodic cycle for 1 week, followed by either ad libitum or time-restricted feeding for 17 to 20 consecutive weeks. Over the final 2 weeks, as depicted in Fig. 1B, the mice received injections of either vehicle, MEL, or C3G. The animals were individually housed in metabolic cages on a time-restricted feeding schedule, as depicted in the schematic. On the eighth day, water intake (A) and urine volume (B) were recorded at 2-hour intervals across a 24-hour period. The data for the 12- hour day, 12-hour night, and full 24-hour periods were pooled and presented graphically. On day 10, the lights were turned off to assess the internal metabolic rhythm. Two days after that, water intake (C) and urine volume (D) were measured under DD conditions at 2-hour intervals. These experiments were conducted in duplicate to establish the weekly pattern, and the results are displayed on the graph as average values. All data are expressed as mean±standard error of the mean (n=6–8 per group). LD, light-dark; AF, ad libitum feeding; DF, daytime feeding; MEL, melatonin; V, vehicle; C3G, cyanidin-3-glucoside; DD, constant darkness.






