Intravesical Instillation of Hyaluronic Acid With Epidermal Growth Factor for Restoring Urothelial Denudation and Alleviating Oxidative Stress in Lipopolysaccharide-Induced Interstitial Cystitis of Rats
Article information
Abstract
Purpose
To investigate the efficacy of an intravesical instillation of hyaluronic acid (HA) combined with epidermal growth factor (EGF) for the treatment of interstitial cystitis (IC) using a lipopolysaccharide (LPS)-induced IC animal model.
Methods
A total of 24 female Sprague-Dawley rats were randomized to 4 groups: sham control, IC, HA, and treatment (HA/ EGF) groups. A polyethylene-50 tube was placed inside the bladder of each animal. IC was induced by twice-weekly instillations of LPS for 3 weeks, which resulted in chronic injury of the urothelium. Animals in the sham control group only received saline instillation. Treatment solutions of HA and HA/EGF were given on days 0, 7, and 14 after IC induction (400 μL of HA in a concentration of 0.4 mg/0.5 mL and 400 μL of NewEpi, a commercialized HA/EGF mixture containing 2 μg of EGF and 0.4 mg of sodium hyaluronate). Animals were sacrificed on day 21 for further examinations.
Results
The HA/EGF group showed visible improvement in hematuria with a significant reduction of red blood cells in the urine compared to the HA group. Histological examination revealed that HA/EGF treatment reversed the abnormalities developed in IC, including infiltration of inflammatory cells, irregular re-epithelialization, and fibrotic tissue. Moreover, HA/ EGF significantly reduced the levels of proinflammation cytokines (tumor necrosis factor-α, interleukin [IL]-6, and IL-1β) and substantially lowered the elevated oxidative stress biomarker malondialdehyde, yet restored the levels of antioxidant enzymes glutathione peroxidase and superoxide dismutase, with superior results than HA treatment. Cystometry studies indicated that HA/EGF significantly prolonged intercontraction interval and increased micturition volume.
Conclusions
HA/EGF has been demonstrated as a more effective treatment for enhancing the urothelium lining and reducing inflammatory changes to alleviate clinical symptoms associated with IC in rats, compared to HA alone.
• HIGHLIGHTS
- This study demonstrates that, compared to hyaluronic acid treatment alone, incorporating epidermal growth factor significantly alleviates the symptoms of interstitial cystitis and enhances healing by reducing inflammatory cytokines, correcting the levels of oxidative stress-related biomarkers, and restoring bladder function.
INTRODUCTION
As per the guidelines provided by the American Urology Association (AUA) guideline, interstitial cystitis (IC) is a chronic condition characterized by uncomfortable symptoms, including pain associated with the urinary bladder (UB), often accompanied by lower urinary tract symptoms such as urgency and frequency [1]. Before reaching a diagnosis, it is essential to rule out any identifiable factors, such as infection, and the symptoms should be persistent for a minimum of 6 weeks. Currently, the underlying pathophysiology of the condition remains unknown [1], and there is no consensus regarding the specific pathological features required for diagnosis. The ultimate goal of treatment is to improve the general quality of life (QoL) [1]. Although the exact pathophysiology of IC remains elusive, several prominent features have been extensively discussed under microscopy, which include mast cells’ infiltration, urothelial denudation, and neuronal hypersensitivity [2-4]. In addition to these factors, neurogenic inflammation and autoimmune response have also been proposed as potential contributors to the development of IC [5, 6].
When it comes to treating uncomplicated IC and IC without Hunner lesions, behavioral treatments play a pivotal role and are considered essential nonpharmacological options. Regarding oral medicines, certain remedies such as amitriptyline or hydroxyzine have shown potential to offer temporary symptomatic relief. With intravesical instillation, commonly used substances include dimethyl sulfoxide (DMSO), heparin, or lidocaine [1].
In the pathological features of IC, urothelial denudation refers to the disruption of the mucin glycosaminoglycan (GAG) layer lining at the surface of the urothelium. The deficiency of this layer may allow irritant chemicals in the urine to permeate into the underlying structures, leading to symptoms. The supplementation of hyaluronic acid (HA), one of the key components involved, has been demonstrated to improve subjective parameters such as QoL and objective parameters such as voiding volume [7]. The epidermal growth factor (EGF) was proposed to play a role in regenerating the human urothelium. There is abundant expression of receptors for EGF in UB, and the EGF was mainly secreted from the kidney and urothelium in UB [8-10]. Following mucosal injury, heparin-binding EGFlike growth factor (HB-EGF), a glycoprotein consisting of 76– 86 amino acids, was found to reconstruct the urothelium via autocrine in the UB [11]. In inflammation, it could mediate the actions of several cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6 [12]. In addition, HB-EGF could also be a protective shield against elevated oxidative stress (OS), such as upregulated lipid peroxidation [13].
Although there was a theoretical basis for HA and EGF, the intravesical instillation of HA/EGF has not been included in the AUA guideline. Moreover, there was no recent discussion in the literature across major databases. This research aims to contribute the latest information regarding the efficacy of intravesical instillation of HA/EGF in treating IC using an animal model.
MATERIALS AND METHODS
Animals
We used adult female Sprague‐Dawley rats (Bio Lasco Animal Center, Taipei, Taiwan) weighing between 200 and 300 g. The animals were housed in a room maintained at 22°C–24°C and 50%–60% relative humidity, with an alternating 12‐hour lightdark cycle and ad libitum food and water supply. The Committee of Animal Experiments at Taipei Veterans General Hospital (Taipei, Taiwan) approved all animal procedures, and we adhered to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA).
Rats Model Establishment
Anesthesia was induced by the intramuscular injection of 16 mg/kg of xylazine (Rompun, Bayer, Taipei, Taiwan) and 0.04 mg/kg of zolazepam plus tiletamine (Zoletil, Virbac, Carros, France). The UB was exposed after making a vertical incision to the lower abdomen and another at the bladder dome area. Next, we placed one end of a polyethylene-50 tube (BD Intramedic, BD, Sparks, MD, USA) inside the bladder and closed the bladder with a watertight suture. The contralateral end of the polyethylene tube ran through the subcutaneous layer of the right flank area and out through the posterior neck area. After checking the urine drainage without obstruction or leakage through the bladder incision site, we fixed the polyethylene-50 tube and closed the abdominal wound. An intramuscular injection of 15 mg/kg of cefprozil was administered to prevent periprocedural infection. We kept the animals in warm cages individually for close observation until they woke up completely. A total of 24 female rats were divided into 4 groups: sham control (Sham), LPS-induced IC (LPS-IC), LPS-induced IC treated with HA (LPS-HA), and LPS-induced IC treated with HA and EGF (LPS-HA/EGF) group.
Induction of lipopolysaccharide (LPS)‐induced cystitis and HA/EGF administration
An exteriorized catheter, entering the dome of the UB, was placed at the back of the neck. We induced IC with 150 μL of purified LPS obtained through phenol extraction (1 μg/μL) (E. coli O55:B5, Sigma-Aldrich, St. Louis, MO, USA). Twice-weekly instillations of LPS through the catheter for 3 weeks induced a long-lasting and chronic injury to the urothelium. Animals in the sham control group only received sterilized saline instead of LPS.
HA (Mylan Institutional, Coill Rua, Inverin, County Galway, Ireland) was obtained at a concentration of 0.4 mg/0.5 mL. Animals in the HA group received 400 μL of HA intravesical instillation on days 0, 7, and 14 after IC induction. The HA/EGF treatment solution was prepared using 400 μL of NewEpi (a commercialized mixture of HA/EGF containing 2 μg of EGF and 0.4 mg of sodium hyaluronate; Good-Care Biotech Ltd., Taipei, Taiwan), and it was administered following the same schedule.
On day 21, rats were sacrificed to collect various parameters from the urothelial tissues in UB. Four days after IC induction and before sacrificing the animals, we collected 24-hour urine samples from all rats using metabolic cages to assess the color and presence of blood in the urine.
Cystometric Investigations
Prior to sacrifice, cystometric measurements were conducted on 4 groups of unanesthetized and unrestricted rats. For the cystometry procedure, a polyethylene-50 tube was inserted into the bladder and connected via a T-tube to a pressure transducer (SP844 pressure transducer, MEMSCAP, Bernin, France) and a syringe pump (R462; RWD, Guangdong, China). A continuous infusion of Normal Saline (0.9 % NaCl) into the UB was maintained at a steady rate of 0.4 mL/min. micturition volume (MV) was measured using an electronic precision balance (ViBRA AB-623; SHINKO DENSHI Co., Tokyo, Japan) connecting to a urine collector. The bladder pressure and MV were continuously recorded for a minimum duration of 1 hour using the Powerlab system (AD Instruments, Dunedin, New Zealand) with LabChart software. Various parameters, including basal pressure (cm H2O), micturition pressure (cm H2O), and micturition interval (MI) (minutes), were obtained throughout the cystometric procedure. Following the completion of cystometric investigations, the rats were anesthetized as described above, and their UBs were subsequently removed. Finally, the rats were euthanized by inhalation of diethyl ether.
Histological Examinations
The excised UB was fixed in 4% paraformaldehyde on day 21 after the first LPS injection. On day 21, following the initial saline injection, UB tissues were collected from the rats in the sham control group. They were frozen, cut on a cryostat at 5‐μm thickness, and stained with hematoxylin and eosin (H&E). To further assess specific parameters, we employed cytokeratin immunostaining (Keratin, Pan Ab-1; Thermo Scientific, Foster City, CA, USA) to evaluate urothelial denudation, toluidine blue staining (Toluidine blue-O; Daejung Chemicals and Metals, Seoul, Korea) to assess mast cell infiltration, Masson trichrome staining (Junsei Chemical, Tokyo, Japan) to examine tissue fibrosis, and Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling assay to (Roche, Mannheim, Germany) detect apoptosis.
Measurement of Inflammatory Cytokine and Oxidative Marker Concentrations
On day 21, the UB tissues were homogenized with 100 μL ice-cold tissue radio-immunoprecipitation assay lysis buffer. The homogenate was incubated for 30 minutes and centrifuged at 12,000 g for 20 minutes at 4°C. The supernatant’s total protein concentration was assessed using a bicinchoninic acid assay kit. The TNF-α, IL-6, and IL-1β levels in UB homogenates were determined using rat enzyme-linked immunosorbent assay (ELISA) kits (BioLegend, San Diego, CA, USA). We used the NE-PER Nuclear and Cytoplasmic Extraction system (Thermo Fisher Scientific, Waltham, MA, USA) to prepare nuclear extracts of the supernatants.
Statistical Analysis
Experimental data are presented as the mean±standard deviation. Differences between multiple groups in 1-way analysis of variance (ANOVA) were determined using Tukey honestly significant difference test in GraphPad Prism (ver. 8; GraphPad Software, La Jolla, CA, USA). A significance level (α) of 5% was used as the threshold to determine statistical significance.
RESULTS
HA/EGF Improved the Hematuria and Histological Pathologies in LPS-Induced IC
In terms of the visual appearance of urine samples, we observed an improvement in hematuria following the HA/EGF instillation treatment. Furthermore, only the HA/EGF treatment demonstrated a significant reduction in the level of red blood cells in the urine. In contrast, treatment with HA alone did not effectively improve hematuria (Fig. 1). Upon histological assessment, it was evident that the HA/EGF treatment effectively ameliorated the pathological changes observed in rats with LPS-induced IC. The H&E examination revealed the presence of mixed inflammatory cell infiltration and abnormal thickening of the reepithelium in LPS-induced IC (Fig. 2). Additionally, Masson trichrome staining demonstrated an augmented tissue fibrosis in LPS-induced IC. All of these abnormalities were effectively reversed through intravesical instillation of HA/EGF, demonstrating superior outcomes in comparison to HA-only treatment (Fig. 2).
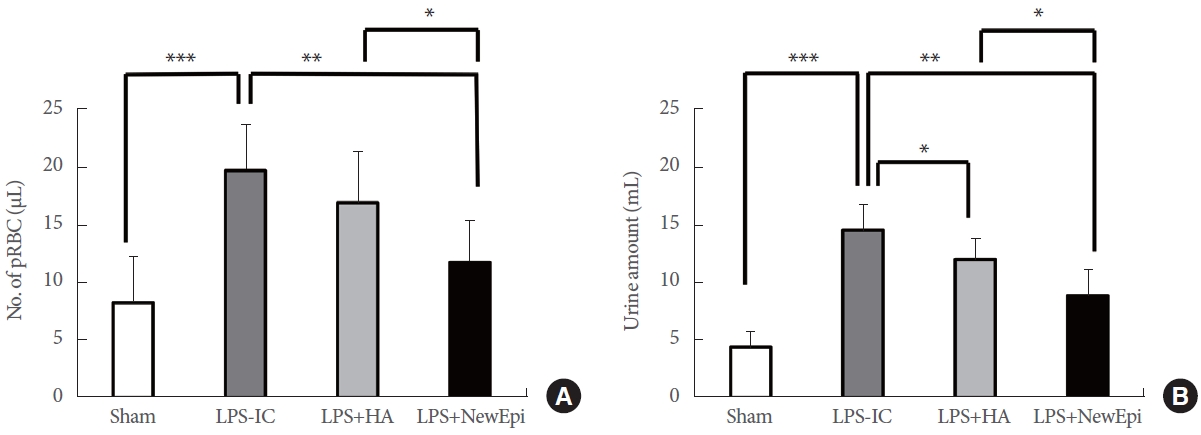
Hematuria measurements (A) and total daily urine amount (B). pRBC, packed red blood cell; LPS, lipopolysaccharide; IC, interstitial cystitis; HA, hyaluronic acid. NewEpi (Good-Care Biotech Ltd., Taipei, Taiwan). *P<0.05. **P<0.01. ***P<0.001.
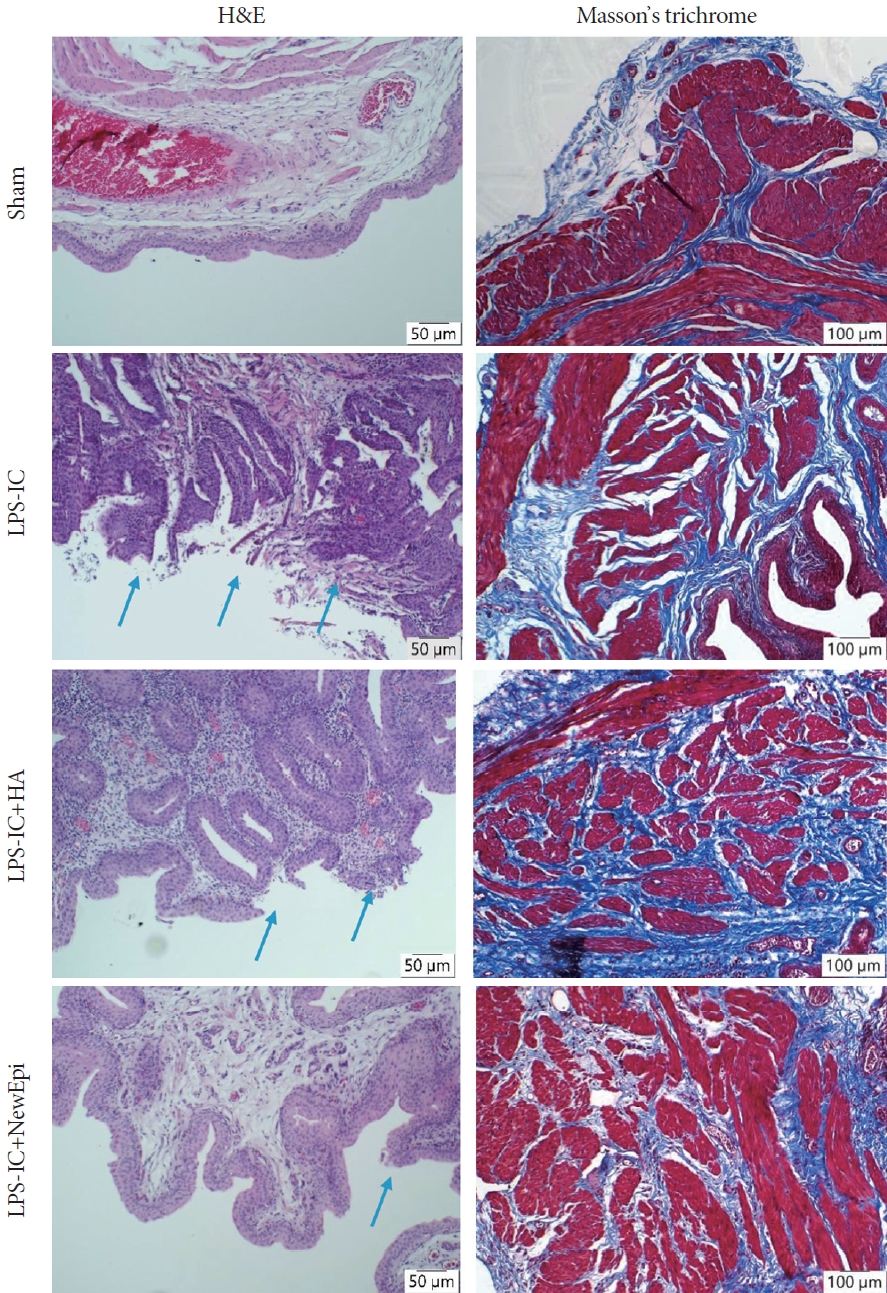
Hematoxylin and eosin (H&E) stains: the tissues of LPS-induced IC exhibited a disruption in the typical architecture of the urothelium, accompanied by an increase in mast cells. Following treatment with HA or NewEpi (Good-Care Biotech Ltd., Taipei, Taiwan), the tissues regained the characteristic urothelial architecture. Blue arrows marked the urothelial denudation. Masson trichrome showed less fibrosis in rats treated with NewEpi. LPS, lipopolysaccharide; IC, interstitial cystitis; HA, hyaluronic acid.
HA/EGF Decreased Inflammatory Cytokines in UB Tissues of LPS-Induced IC
The levels of inflammatory biomarkers TNF-α, IL-6, and IL-1β were assessed in UB tissues using ELISA. The expressions of these biomarkers were much higher in the IC group compared to the sham control group (Fig. 3). After intravesical instillation of HA/EGF, the levels of inflammatory cytokines remarkably decreased (Fig. 3). The extent of improvement was significantly greater in the HA/EGF group compared to the group receiving HA treatment alone. TNF-α is the only cytokine that was significantly reduced by HA-only treatment. In contrast, HA/EGF treatment exhibited significantly superior outcomes compared to HA treatment alone for all 3 cytokines: TNF-α, IL-6, and IL-1β.

Down-regulation of inflammatory cytokines by HA and NewEpi (Good-Care Biotech Ltd., Taipei, Taiwan): TNF-α (A), IL-6 (B), and IL-1β (C). TNF, tumor necrosis factor; IL, interleukin; LPS, lipopolysaccharide; IC, interstitial cystitis; HA, hyaluronic acid. NewEpi (Good-Care Biotech Ltd., Taipei, Taiwan). *P<0.05. ***P<0.001.
HA/EGF Triggered Antioxidative Effects in UB Tissues of LPS-Induced IC
We examined the levels of OS biomarkers, including malondialdehyde (MDA), glutathione peroxidase (GSH-Px), and superoxide dismutase (SOD), in the UB homogenates. The established IC model demonstrated a significant elevation in MDA concentrations and depletion of GSH-Px and SOD in UB tissues following LPS administration (Fig. 4). The elevated MDA level was substantially lowered by HA/EGF treatment, more so than by HA alone. HA/EGF was the only treatment that restored the activities of the antioxidant enzymes GSH-Px and SOD in UB tissues, which were depleted by LPS (Fig. 4).
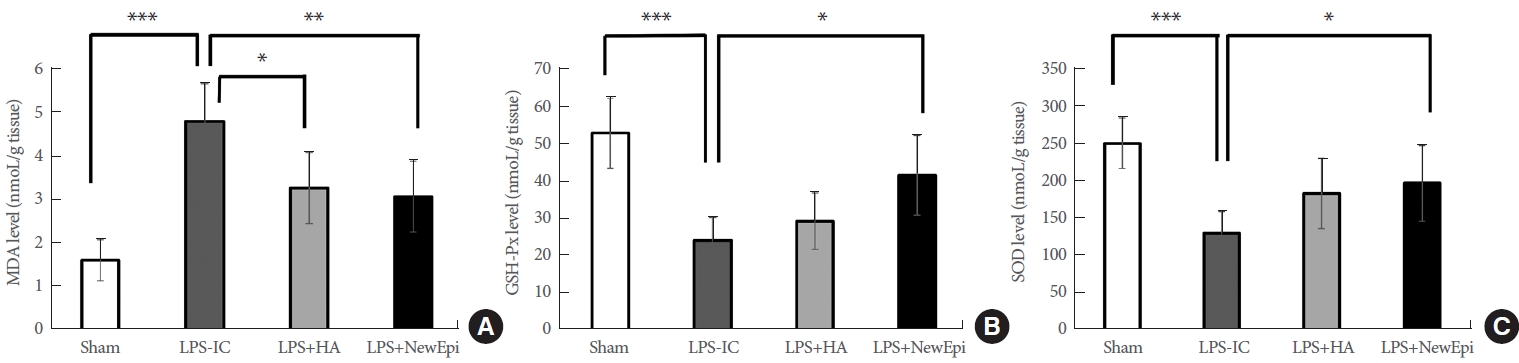
The oxidative stress biomarkers: malondialdehyde (MDA; A), glutathione peroxidase (GSH-Px; B), and superoxide dismutase (SOD; C). As compared to Sham subgroups, elevating MDA, increasing GSH-Px and SOD were displayed in LPS-IC group. NewEpi (Good-Care Biotech Ltd., Taipei, Taiwan) suppressed the MDA level but increased GSH-Px and SOD levels. LPS, lipopolysaccharide; IC, interstitial cystitis; HA, hyaluronic acid. *P<0.05. **P<0.01. ***P<0.001.
HA/EGF Prolonged Intercontraction Interval and Increased MV in the UB of LPS-Induced IC
Cystometry was conducted to assess the functional improvement across the different groups. Compared to the sham and HA/EGF group (Fig. 5), rats with LPS-induced IC exhibited a significant reduction in both the mean irregular voiding pattern and the mean MI (IC=91.10±14.63 seconds; 137.91±15.61, 143.25±41.26, and 162.75±54.87 in Sham, HA, and HA/EGF respectively; F [3, 20]=3.566, P<0.05). According to the Tukey post hoc test, the intercontraction interval in the LPS-IC model showed significant improvement only in the group treated with HA/EGF (P<0.05).
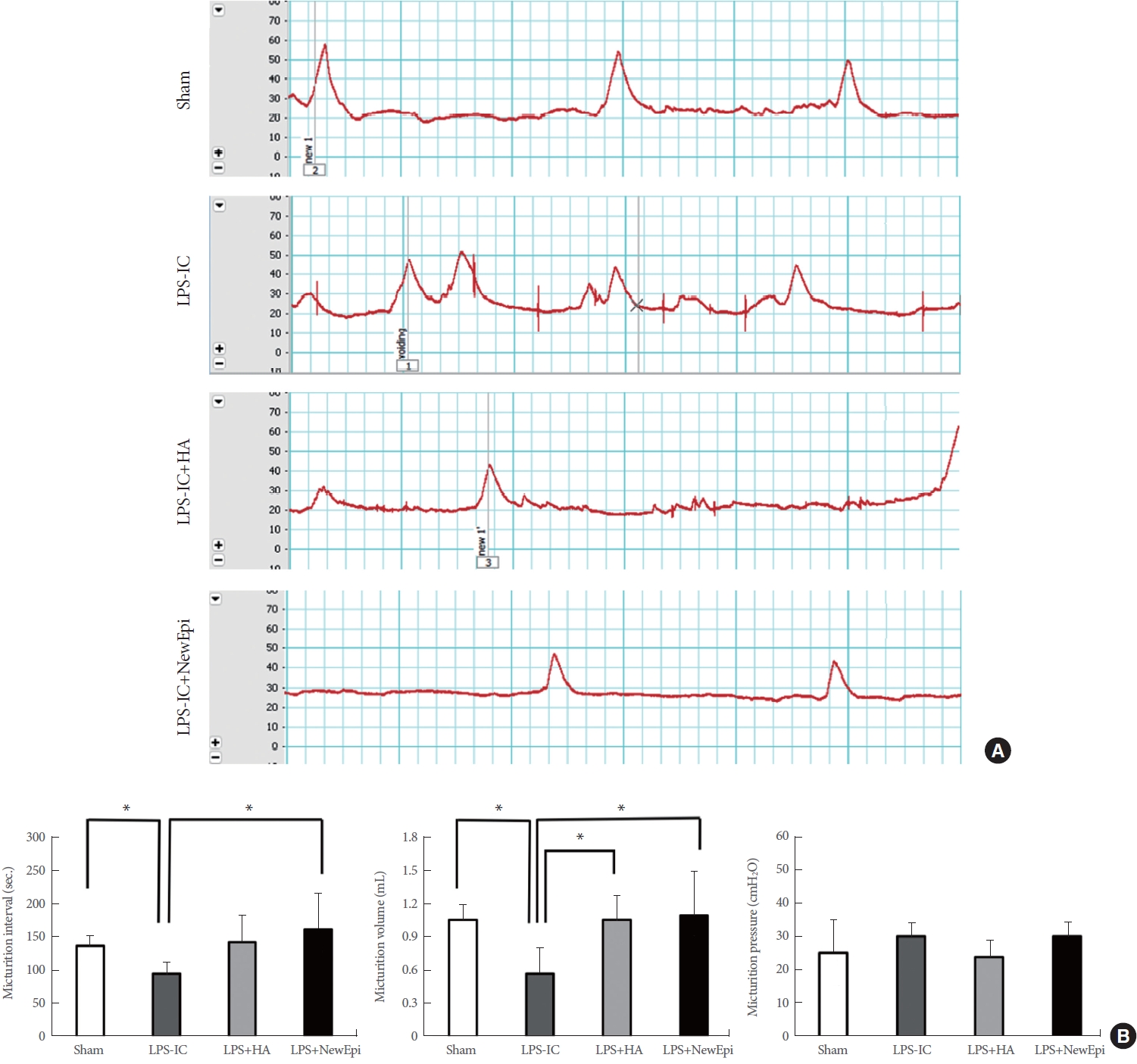
Urodynamic testing and parameters. (A) Cystometriograms. (B) Comparison of cystometric parameters among groups. LPS, lipopolysaccharide; IC, interstitial cystitis; HA, hyaluronic acid. NewEpi (Good-Care Biotech Ltd., Taipei, Taiwan). *P<0.05.
Both the HA and HA/EGF treatments demonstrated comparable significant improvements in MV (1.067±0.216 mL, P<0.05; 1.100±0.405 mL, P<0.05, respectively) (Fig. 5). Finally, ANOVA test revealed no significant differences micturition pressure among the groups (F [3, 20]=1.559, P=0.23).
DISCUSSION
In the early studies investigating molecular changes in IC, researchers analyzed changes in biomarkers by collecting urine samples, allowing for the detection of initial alterations associated with IC [14, 15]. Previous results have shown a significant elevation in EGF levels in urine samples, indicating the removal of proliferative factors from the urothelium in IC, particularly in symptomatic cases [15]. The current AUA guideline includes intravesical instillation therapies, including DMSO, heparin, and lidocaine, but without Grade-A evidence supporting their use [1].
HA has been suggested as a supplement to enhance the GAG lining in the urothelium, with the ability to improve the symptoms of IC [16-18]. In a meta-analysis, several clues have emerged suggesting that combining HA with other agents, such as chondroitin sulphate, may be more effective in improving subjective symptoms than using HA alone [19]. However, the combination of HA with EGF has not been examined in an randomized controlled trial setting before.
The urothelial architecture is a complex structure comprised of multiple layers, each with its own distinct morphology and functions. Under normal circumstances, the turnover rate of urothelial tissue is relatively slow, taking around 6 to 12 months for complete renewal. However, in response to injuries, the urothelium can initiate a rapid renewal process [20]. This suggests the presence of stem cells within specific layers of the urothelium that are capable of regenerating and replacing damaged or deteriorated cells in response to microenvironmental changes. A recent study observed that exogenous EGF could stimulate urothelial regeneration and migration in a dose-dependent fashion and combination with the other ingredients [21]. The underlying mechanism was proposed to involve the EGFR/MAPK/SOX9 pathway [22]. Thus, we embraced the idea of EGF recombination and assessed the effectiveness of an existing EGF product in treating IC, focusing on its healing properties [21].
As mentioned earlier, HA exhibited increased effectiveness in treating IC when combined with other ingredients [19]. However, the combination of HA and EGF (HA/EGF) has not been studied explicitly concerning IC treatment. In this experiment, we observed significantly reduced red blood cells in the urine samples of HA/EGF group. Upon microscopic examination, we discovered that the HA/EGF restored the damaged urothelium caused by LPS instillation and effectively modulated the infiltration of mast cells. In a previous study, significant alterations were identified in TNF-α, IL-1β, and IL-6, within urine samples collected from individuals with IC [23]. Consistent with these findings, our study also observed a noticeable decrease in the levels of these cytokines and chemokines. Certain urine OS markers have emerged as potential indicators for the detection of IC [24]. In cellular physiology, increased OS leads to mitochondrial lay-off, and apoptosis ensues due to a lack of energy. Yang et al. [25] demonstrated that reducing OS levels could potentially benefit the apoptotic condition associated with IC. In our study, the administration of HA/EGF resulted in a significant reduction in the peroxidative unsaturated lipid product MDA, indicating an effective reduction in OS. Additionally, HA/EGF treatment increased the levels of antioxidants SOD and GSH-Px, suggesting an enhancement of the antioxidant defense system. Notably, HA/EGF treatment exhibited superior positive outcomes in comparison to HA treatment alone. This capability suggests that HA/EGF can restore the damaged urothelium by reducing OS levels and preventing subsequent urothelial apoptosis.
Our cystometry studies revealed that only the HA/EGF treatment prolonged the MI, while both HA and HA/EGF treatments significantly increased MV. Similarly, in a previous study [26], urodynamic testing demonstrated comparable therapeutic effects of cis-urocanic acid and HA in improving bladder function in a hydrochloric acid-induced acute bladder inflammation rat model. To date, HA appears to be an established protective agent in the restoration of bladder epithelium. Our findings suggest that the combination of HA and EGF may yield superior outcomes in restoring bladder function compared to HA-only treatment.
This study has several limitations. Firstly, the investigational agent NewEpi is a commercially available mixture with a fixed composition of HA and EGF, which restricts the ability to test the optimal therapeutic dose. Secondly, it is acknowledged that including a group solely treated with EGF would be appropriate to assess the efficacy of the target therapy. Unfortunately, due to constraints, an EGF-only treatment group could not be included in the present study. However, an ongoing experiment is being conducted to evaluate the effects of HA and EGF separately, which may provide further insights.
Notes
Grant/Fund Support
This research was sponsored by the academic funds of Yen Tjing Ling Medical Foundation (Fund Ref ID: CI-112-17).
Research Ethics
The study was performed according to the Committee of Animal Experiments at Taipei Veterans General hospital (Taipei, Taiwan) approved all animal procedures, and we adhered to the Guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health (Bethesda, MD, USA).
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: CCL, HLL
· Data curation: CCL, JMY, THH, HLL
· Formal analysis: CCL, JMY, THH, HLL
· Funding acquisition: CCL, HLL
· Methodology: CCL, THH
· Project administration: CCL, THH
· Visualization: THH
· Writing - original draft: CCL, HLL
· Writing - review & editing: JMY
