A Randomized, Double-Blind, Placebo-Controlled, Bridging Study to Evaluate the Efficacy and Safety of Vibegron in Treating Korean Patients With Overactive Bladder
Article information
Abstract
Purpose
Vibegron, a novel, potent β3 agonist, has been approved for clinical use in overactive bladder (OAB) treatment in Japan and the Unites States. We performed a bridging study to investigate the efficacy and safety of a daily 50-mg vibegron (code name JLP-2002) dose in Korean patients with OAB.
Methods
A multicenter, randomized, double-blind, placebo-controlled study was conducted from September 2020 to August 2021. Adult patients with OAB with a symptom duration of more than 6 months entered a 2-week placebo run-in phase. Eligibility was assessed at the end of this phase and selected patients entered a double-blind treatment phase after 1:1 randomization to either the placebo or vibegron (50 mg) group. The study drug was administered once daily for 12 weeks and follow-up visits were scheduled at weeks 4, 8, and 12. The primary endpoint was the change in mean daily micturition at the end of treatment. The secondary endpoints included changes in OAB symptoms (daily micturition, nocturia, urgency, urgency incontinence, and incontinence episodes, and mean voided volume per micturition) and safety. A constrained longitudinal data model was used for statistical analysis.
Results
Patients who took daily vibegron had significant improvements over the placebo group in both primary and secondary endpoints, except for daily nocturia episodes. The proportions of patients with normalized micturition and resolution of urgency incontinence and incontinence episodes were significantly higher in vibegron group than in the placebo. Vibegron also improved the patients’ quality of life with higher satisfaction rates. The incidence of adverse events in the vibegron and placebo groups was similar with no serious, unexpected adverse drug reactions. No abnormality in electrocardiographs was observed as well as no significant increase in postvoid residual volume.
Conclusions
Once daily vibegron (50 mg) for 12 weeks was effective, safe, and well-tolerated in Korean patients with OAB.
INTRODUCTION
Overactive bladder (OAB) is a symptomatic diagnosis defined as “urinary urgency,” usually accompanied by increased daytime frequency and/or nocturia, with or without urgency incontinence (OAB wet and OAB dry, respectively) in the absence of urinary tract infection or other pathologic conditions by the International Continence Society [1]. OAB is a chronic disease with an estimated prevalence of 8.0%‒16.5% in adults, with similar rates found in men and women [2]. The mainstay of OAB treatment is pharmacotherapy [3] for which anticholinergics have traditionally been widely used. The problem with anticholinergics is common side effects, such as dry mouth, constipation, and dry eye, that lead to decreased compliance of patients [4]. Moreover, it has been reported that long-term prescription of anticholinergics is associated with cognitive dysfunction or dementia in older patients [5]. Considering the chronic nature of OAB, patients’ persistence and compliance with the treatment are major implications for improving prognosis and outcome.
Mirabegron, the first U.S. Food and Drug Administration (FDA)-approved β3 agonist for treating OAB, has been the only available drug for patient’s intolerant to anticholinergics. Compliance to mirabegron is reported to be higher than that of anticholinergics due to different in possible side effects and their intensity [6,7]. In addition, combination therapy with various anticholinergics has expanded possible treatment options. Vibegron, a novel, potent, and selective β3-adrenoceptor agonist, has proved its efficacy in treating OAB in multiple clinical trials and was first approved for clinical use in Japan (50 mg) in September, 2018 [8]. Furthermore, the FDA approved vibegron (75 mg) for treating OAB in December, 2020 [9]. Meanwhile, there has been no clinical trials investigating the safety and efficacy of vibegron in the Korean population. The objective of this bridging study was to evaluate the safety and efficacy of a 50-mg dose of vibegron (code name JLP-2002) daily in Korean patients with OAB.
MATERIALS AND METHODS
Study Design
This was a multicenter, parallel-group bridging study in patients with OAB. The study was conducted at 20 sites throughout Korea from September 2020 to August 2021 and consisted of 2 phases: a 2-week, single-blind, placebo run-in phase and a 12-week, double-blind treatment phase. Adult patients with OAB who met the selection criteria [10] and had symptoms lasting for more than 6 months entered the first phase and were prescribed a placebo once daily. At the end of the run-in phase, information from each patient’s 3-day voiding diary was used to determine eligibility as follows: daily micturition ≥8, either urgency episodes/day ≥1 or urgency incontinence episodes/day ≥1, and the total of urgency incontinence episodes over the half of incontinence episodes. Patients with polyuria (total daily urine output ≥3,000 mL), postvoid residual volume (PVR) ≥100 mL, adherence <75% during the placebo run-in phase, high blood pressure (160/100 mmHg) or baseline pulse 110 bpm were excluded from randomization. Selected patients were randomly assigned to either the placebo or the vibegron (50 mg) group in a 1:1 ratio with adjustment for sex, baseline mean daily micturition, and presence of urgency incontinence. The respective drug was administered once daily for 12 weeks, and follow-up visits were scheduled at 4, 8, and 12 weeks after the start of the treatment phase. The overall study design is summarized in Supplementary Fig. 1 and the exclusion criteria [10] from a previous Japanese clinical trial was applied.
Efficacy and Safety Assessments
The drug efficacy assessment consisted of changes in voiding profiles and scores of validated questionnaires regarding subjective symptoms, quality of life, and satisfaction with the treatment. At each scheduled visit, patients were asked to submit a completed 3-day voiding diary and overactive bladder symptoms score (OABSS). Quality of life was evaluated using the King’s Health Questionnaire at weeks 0 and 12. The patients’ satisfaction with the treatment was assessed with the Patient Global Impression (PGI) scale at week 12. The level of satisfaction was based on a 7-point scale; very much improved (1), much improved (2), minimally improved (3), no change (4), minimally worse (5), much worse (6), and very much worse (7). A PGI ≤3 was defined as ‘effective improvement’ and a PGI ≤2 was defined as ‘significant improvement.’ Safety was assessed with adverse events (AEs), vital signs, physical examinations, clinical laboratory tests, 12-lead electrocardiogram results, and postvoid residuals. The severity of any AEs was based on the Common Terminology Criteria for Adverse Events (CTCAE version 5.0).
The primary endpoint of the study was the change in mean micturition per day at week 12 from baseline. The secondary endpoints included changes in mean daily micturition, nocturia episodes, urgency, urgency urinary incontinence, incontinence episodes, mean voided volume, normalization of the aforementioned OAB symptoms, and OABSS at each visit, along with quality of life and satisfaction at the end of treatment. The normalization of each OAB symptom was defined as mean daily micturition <8/day; nocturia episodes <1/day; and no evidence of urgency, urgency urinary incontinence, and incontinence episodes, respectively.
Statistical Analysis
We referred to the mean and standard deviation of changes in mean micturition episodes per day at week 12 from a Japanese phase 3 study [10] to determine the size of our study population. A sample size of 84 patients in each group provided 80% power to demonstrate the superiority of vibegron over the placebo with a 2-tailed significance level of 0.05. The minimum number of required patients was 168, and considering a dropout rate of approximately 20%, a total sample size of 210 patients (105 per group) was obtained for the treatment phase.
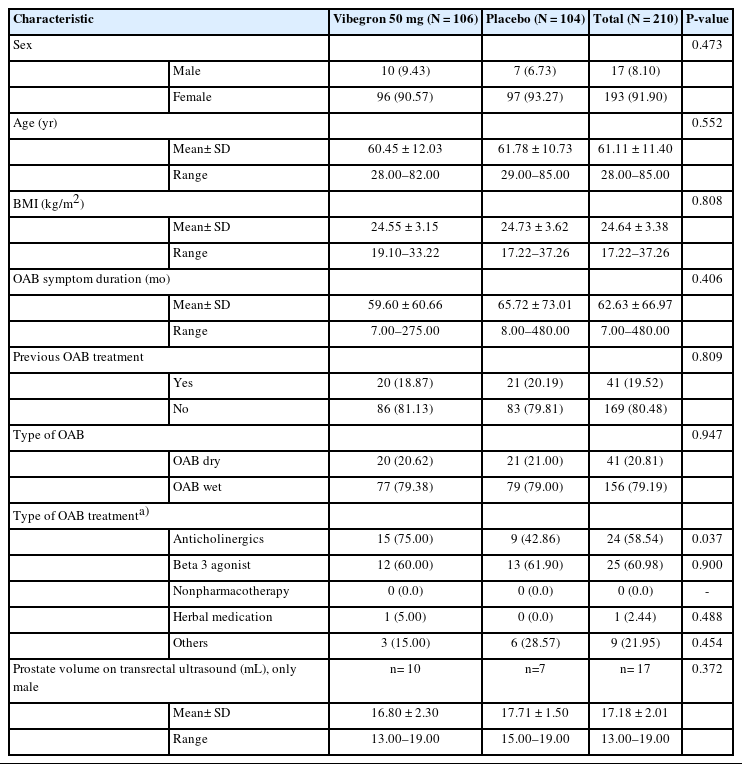
Baseline characteristics and demographics of the study population were assessed in a randomized set, involving all patients who were allotted to the treatment phase. Safety was analyzed in a safety set including all patients who took the study drug at least once during the treatment phase. Efficacy analysis was performed primarily in the full analysis set (FAS) and secondarily in a per-protocol set (PPS), a subset of the FAS. The FAS consisted of the safety set patients with available efficacy measurements, and the PPS included patients who completed the study without any major violation of the study protocol.
Statistical analysis was performed with SAS ver. 9.4 (SAS Institute, Cary, NC, USA). The least squares (LS) mean and 2-tailed 95% confidence interval of each continuous variable from baseline to the time of assessment was calculated with a constrained longitudinal data analysis model. A P-value < 0.05 was considered statistically significant. Categorical variables were compared with a chi-square test or Fisher exact test.
RESULTS
Baseline Characteristics and Patient Disposition
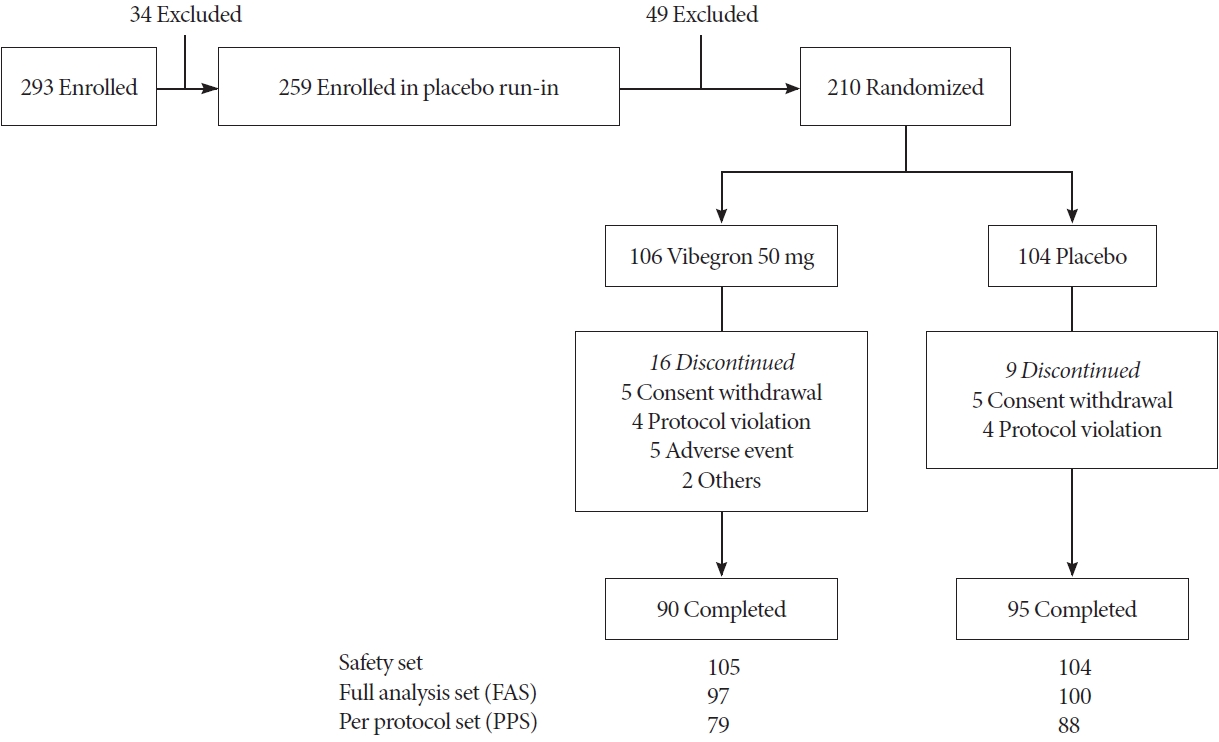
A total of 293 patients were screened, of which 210 were selected and randomized for the study. One patient was excluded from safety analysis due to nonadministration of the study drug. The FAS population was 197 after excluding 11 patients without efficacy data and one patient with dual enrollment (Fig. 1). Baseline characteristics in the randomized set were similar between the groups (Table 1). Upon completion of the study, the proportions of patients with a drug compliance ≥75% during double-blind treatment phase was 94.29% in the vibegron group and 100% in the placebo group. Furthermore, the mean exposure duration to vibegron and the placebo was 77.20± 20.99 and 81.68±12.50 days, respectively.
Efficacy
Compared to the placebo, vibegron significantly improved both the primary and secondary efficacy endpoints, except for nocturia episodes, by week 12. Moreover, changes in LS mean±standard deviation for the primary endpoint was -2.38±0.39 in the vibegron group and -1.21±0.40 in the placebo group (P=0.0021), suggesting the efficacy of vibegron was superior to that of the placebo. In addition, over the 12 weeks, significant improvements in the LS means of daily urgency, urgency incontinence, incontinence episodes, and voided volume per micturition were seen in the vibegron group (Table 2).
At week 4, daily micturition, urgency episodes, voided volume per micturition and total OABSS score were significantly improved from the initiation of vibegron treatment. At week 8, improvements in all OAB symptoms on voiding diary were superior to those of placebo except for nocturia episodes and the therapeutic efficacy was sustained in all parameters. Specifically, the efficacy of vibegron was superior to that of the placebo at each visit based on daily micturition, urgency episodes, voided volume per micturition, and OABSS (Fig. 2). The proportions of patients with normalized micturition and resolution of urgency urinary incontinence and incontinence episodes were higher in vibegron group than in the placebo group, but a significant difference was not observed in urgency and nocturia episodes (Fig. 3). In addition, vibegron significantly improved the total quality of life score, especially in the domains of incontinence impact and emotions (Supplementary Table 1). In the PGI assessment, 88.24% of patients were satisfied (PGI ≤3) (P=0.0037) and 68.24% of patients were very satisfied (PGI ≤2) with the treatment (P<0.0001). Patients’ satisfaction level was higher in the vibegron group than the placebo group (Supplementary Table 2). Similar results were also observed in the PPS analysis.

Changes in voiding parameters from baseline at each visit. The least-square (LS) mean change±standard error of mean (SEM) or 95% confidence interval (CI) from baseline at each visit with respect to mean daily micturition/day (SEM) (A) urgency episode/ day (SEM) (B), urgency incontinence episode/day (SEM) (C), incontinence episode/day (SEM) (D), nocturia episode/day (SEM) (E), voided volume/micturition (95% CI) (F), total overactive bladder symptoms score (OABSS) (95% CI) (G). The differences between vibegron and placebo group were calculated with constrained longitudinal data analysis model which included adjustment factors. *P<0.05. **P<0.001.
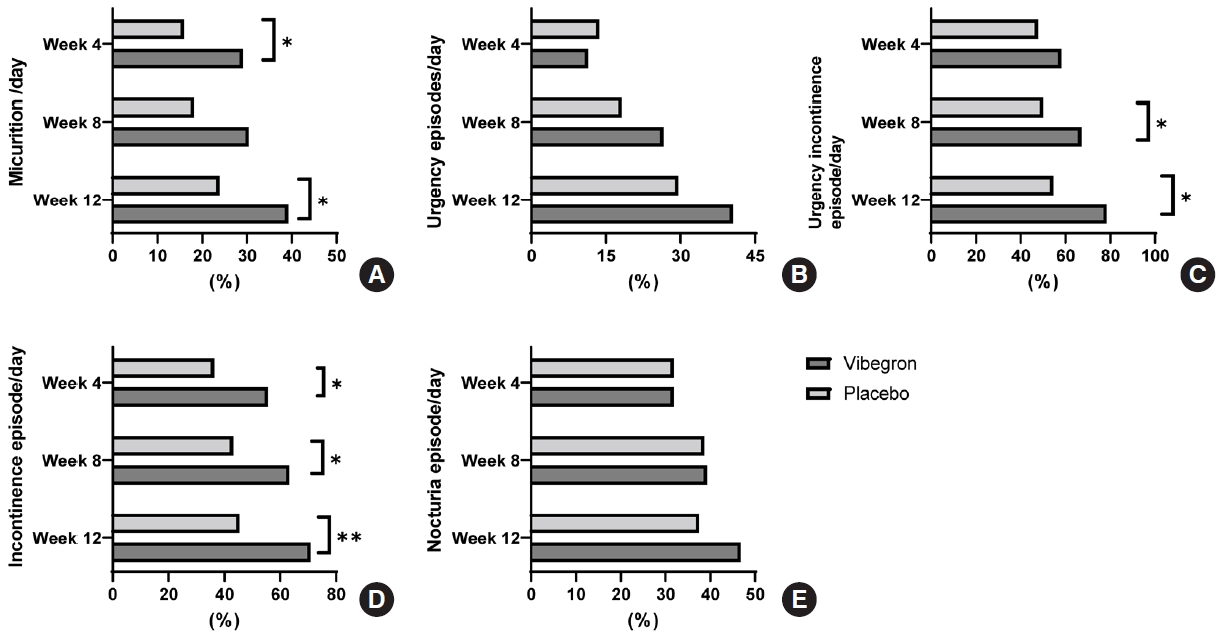
Proportions of normalization or resolution of OAB symptoms. (A) Normalization of daily micturition. (B) Resolution of urgency episodes/day. (C) Resolution of urgency incontinence/day. (D) Resolution of incontinence episode/day. (E) Normalization of nocturia episode/day. Proportion of patients were compared between vibegron and placebo group with chi-square test. *P<0.05. **P<0.001.
Safety
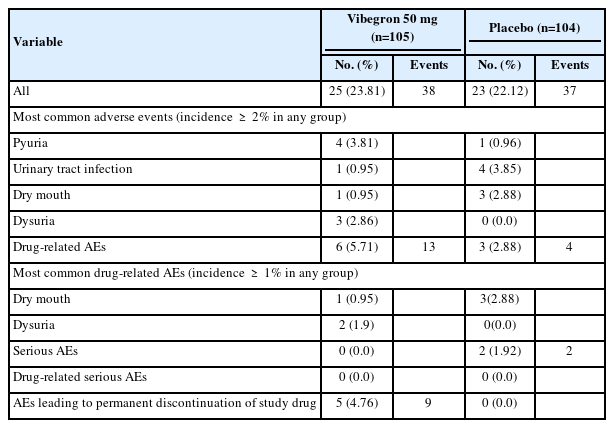
The overall incidence of any AEs was similar between groups (vibegron: 23.81% and placebo: 22.12%). The prevalence of AEs was highest between weeks 4 and 8 (approximately 10% in each group) and lowest after week 12. All AEs in both groups were mild or moderate in intensity and no deaths or serious AEs were reported. The prevalence of potentially drug-related AEs was 5.71% (6 of 105, 13 cases) in the vibegron group and 2.88% (3 of 104, 4 cases) in the placebo group. Regarding relatedness to the drug, 11 cases were possibly related; 2 cases (dysuria and flushing) were probably related to vibegron, and both were expected AEs noted in the Japanese clinical trial [10] as well. A serious AE was only observed in 1.92% (2 of 104, cellulitis and arthritis) of the placebo group and therefore was not related to the study drug (Table 3).
Drug-related AEs leading to permanent discontinuation was reported in 4.76% (5 of 105, 9 cases) of the vibegron group. Most of them were mild in intensity, except face edema, fatigue and dysuria, that occurred in one patient. All patients with drugrelated AEs successfully recovered at the end of the treatment. Some clinically significant changes in vital signs, physical examination, and laboratory tests were observed, including flushing, urinary tract infection, and an increase in alanine aminotransferase, gamma-glutamyltransferase, and alkaline phosphatase levels. These were all mild in intensity and did not require additional intervention, except the urinary tract infection. Hypertension was only observed in 1.92% of placebo group (2 out of 104). No notable changes in electrocardiogram results and heart rate were observed, and there was no significant increase in PVR.
DISCUSSION
This study investigated the efficacy and safety of vibegron in Korean patients with OAB. A single 50-mg dose of vibegron daily for 12 weeks effectively improved the mean daily micturition episodes and other voiding parameters along with subjective symptoms, quality of life, and satisfaction compared to the placebo. The overall incidence of AEs in the vibegron group was similar to that of the placebo group. Drug-related AEs were more frequently observed in the vibegron group; however, most of them have previously been reported, and none of them were serious or severe in intensity.
Several other studies have also investigated the efficacy and safety of vibegron [10-13]. In the phase 3 study by Kyorin Pharmaceuticals [10], a single dose of 50 or 100 mg of vibegron daily successfully improved daily micturition, urgency, urgency incontinence, and incontinence episodes with no significant differences in AEs compared to those in the placebo. The efficacy of 50 or 100 mg of vibegron on urgency incontinence and nocturia was also proved in a post hoc analysis. After the formal launch of vibegron (50 mg) in Japan, an uncontrolled multicenter study investigated its efficacy and safety in real-world settings [14]. Patients with various OAB medication histories were prescribed daily vibegron for 8 weeks. First-line monotherapy with vibegron was effective for controlling OAB symptoms in treatment naive patients as well as in those who switched from anticholinergics or mirabegron to vibegron. As a result, vibegron might be a potential candidate for patients who present suboptimal results when treated with anticholinergics or mirabegron.
In an international phase 3 study performed in western countries (EMPOWUR study) [11], a single 75-mg dose of vibegron daily provided improvements in OAB symptoms, including daily micturition, urgency and urgency incontinence episodes, and voided volume per micturition after 12 weeks as well. Significant improvement was observed at week 2 and maintained throughout the study period. In addition, their patient-reported outcome analysis with OAB-Questionnaire and PGI (composed of 4 domains: severity, frequency, leakage and change) [15], a larger proportion of patients in the vibegron group submitted optimal responses on all PGIs compared with the placebo group. Consistent results were also reported in a 52-week extension study. [12]
The most common drug-related AEs of vibegron were dry mouth and constipation but their incidence was lower than those of imidafenacin [10]. In a 1-year open-label extension study, treatment with either 50 or 100 mg of vibegron was safe and well-tolerated with a comparable incidence of AEs to that of the placebo and without evidence of clinically significant AEs [13]. β3-adrenoceptor agonists are associated with sympathetic control of the relaxation of the detrusor smooth muscle. Although β3-adrenoceptors are predominantly distributed in the urinary bladder, they are also found in the heart, gastrointestinal tract, and adipose tissue. Consequently, the safety analysis of β3 agonists always focuses on possible cardiovascular outcomes including abnormal blood pressure and pulse rate. Thus far, vibegron seems to have minimal impact on cardiovascular parameters [16].
The overall result of this bridging study is similar to that of the Japanese trial, apart from the improvement in nocturia episodes per day at week 12 which was not statistically significant in the present study. Notably, the baseline nocturia episodes per day were similar between the 2 studies’ populations. In addition, differences in daily nocturia episodes after 12 weeks of vibegron treatment were -0.53 in Korean versus -0.58 in Japanese patients. The differences between the vibegron and placebo groups in each study were also similar (-0.16 in the Korean study and -0.11 in the Japanese study; racial difference, P=0.47). Despite the similar degree of improvement in daily nocturia episodes, it seems that statistical significance could not be achieved in Korean study population due to limited sample size.
This new drug needs sufficient comparative verification with existing drugs—antimuscarinics and mirabegron, in terms of efficacy and safety. The approval on vibegron for treating OAB has not been achieved worldwide yet, so literature about reallife experiences and direct comparison with various antimuscarinics are lacking. Currently available studies include randomized controlled trials which set single antimuscarinics as control group along with placebo [10-12,17]. In EMPOWUR study, vibegron achieved numerically better results for average voided volume per micturition, and average number of micturitions, urge incontinence, and urgency episodes per day than tolterodine. In addition, proportions of patients with 75% or more reduction were achieved faster with vibegron [11]. In 52-week extension study, greater reduction in urgency incontinence and total urinary episodes were observed in vibegron group [12]. In Japanese randomized study, imidafenacin was set as a control but analysis of difference between imidafenacin and placebo or vibegron was not performed. A systematic review and metaanalysis of the efficacy and safety of vibegron versus antimuscarinic monotherapy suggested that the therapeutic effect of vibegron was similar to that of anticholinergics, but vibegron does not increase the risk of AEs [18].
The pharmacologic properties of vibegron compared with mirabegron are follow; Vibegron has higher selectivity to β3-adrenergic receptor and faster maximum response than mirabegrone. Moreover, vibegron does not interact with cytochorome P450 that risk of drug-drug interaction is low [19]. Consequently, whether vibegron has superior therapeutic efficacy or lower risk of AEs is of interest among clinicians. Several studies have reported results indirectly comparing the treatment efficacy of vibegron and mirabegron in patients with OAB. In a Japanese retrospective study, the administration of vibegron was less likely to be discontinued due to suboptimal response than that of mirabegron in both neurogenic and nonneurogenic OAB patients [20]. There have been several systematic reviews that indirectly compared the efficacy of vibegron and mirabegron. Improvement in daily micturition was similar, but vibegron was associated with significant improvement in total incontinence episodes at week 4 and week 52 as well as voided volume at week 12 and week 52 [21,22]. The most recent systematic review and network meta-analysis concluded that both mirabegron and vibegron were comparable and welltolerated in OAB patients but vibegron might be more effective than mirabegron in improving mean voided volume per micturition [23]. Moreover, despite lack of available real-word data on persistence, vibegron was assumed to be cost-effective from a commercial payor and Medicare perspective when compared with other oral pharmacologic agents for OAB [24]. However, all of these studies are limited due to the indirect nature of comparison and insufficient information.
Recently, a prospective randomized control study which compared the efficacy and safety of mirabegron versus vibegron in postmenopausal women with treatment naive OAB was reported [25]. Patients were received either drugs for 12 weeks and both were effective in improving OAB symptoms. The drug discontinuation rate due to adverse effects were similar, but specifically, the reasons for discontinuation were constipation (1 and 5 patients), exanthem (2 and 1 patients) in the mirabegron and vibegron group, respectively, difficulty in urination (1 patient in each group), and elevated blood pressure and headache in 1 patient in mirabegron group. In addition, none of the patients experienced urinary retention and no notable changes in PVR volume observed in both groups.
Common concerns with β3-adrenoreceptor agonists are cardiovascular-related side effects, such as palpitation, elevated blood pressure (hypertension), and headache. As vibegron showed no measurable β1-activity and 2% in β2-activity, compared with mirabegron, which showed 3% activity in β1 and 15% activity in β2, it is expected to have less cardiovascular effects than mirabegron [19]. In such context, only one patient in mirabegron had elevated blood pressure and headache in forementioned study [25]. A Japanese post hoc analysis on vibegron 50 mg, 100 mg, or placebo for 12-week demonstrated no cardiovascular AEs including high blood pressure, palpitation, and tachycardia in those with vibegron 50 mg [16]. Future studies with more patients and longer follow-up is warranted.
The main limitation of the present study is the relatively small sample size compared to large-scale clinical trials. This bridging study received formal approval from Korean Ministry of Food and Drug Safety since vibegron was expected to have similar efficacy and safety in the Korean population, with a small ethnic deviation, to the Japanese population considering its pharmacokinetics and pharmacodynamics [26]. The role of vibegron in OAB treatment requires further investigation into the safety outcomes of long-term dosing regimens, efficacy in treating neurogenic OAB specifically, tolerability of combination therapy with anticholinergics, and the direct comparison with mirabegron treatment. In conclusion, 50-mg vibegron once daily for 12 weeks provided superior therapeutic efficacy than placebo for treating Korean patients with OAB. Additionally, vibegron was well-tolerated without major safety issues.
SUPPLEMENTARY MATERIALS
Supplementary Tables 1, 2, and Fig. 1 can be found via https://doi.org/10.5213/inj.2346022.011.
Supplementary Table 1.
Changes in King’s Health Questionnaire domain scores at the end of treatment (full analysis set)
Supplementary Table 2.
Patient Global Impression (PGI) at the end of treatment
Supplementary Fig. 1.
Schematic diagram of study design.
Notes
Grant/Fund Support
Funding for the study was provided by Jeil Pharm.
Research Ethics
The study was approved by the Institutional Review Board of each center and was performed in compliance with the Korean Good Clinical Practice guidelines, International Council for Harmonization, and Declaration of Helsinki. The use of JLP-2002 was approved by the Korean Ministry of Food and Drug Safety and the protocol was registered on ClinicalTrials.gov (NCT04917315). Informed consent was acquired from all patients in written form before initiation of any procedures related to the study.
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
AUTHOR CONTRIBUTION STATEMENT
· Conceptualization: SJJ, SK, CYO, KJC, DGS, THK, JK, JS, WJB, KL, MC
· Data curation: SJJ, SK, CYO, KJC, DGS, THK, JK, JS, WJB, KL
· Methodology: SJJ
· Project administration: SK, CYO, KJC, DGS, THK, JK, JS, WJB, KL, MC
· Writing - original draft: JHS
· Writing - review & editing: JHS, KL, MC
Acknowledgements
We appreciate the contributions of all the participants and study investigators including Youngseop Chang, Hana Yoon, Jong Bo Choi, Young Sam Cho, Jae Hyun Bae, Joonhwa Noh, Jang-Hwan Kim, Eun Sang Yoo and Sung-Yong Cho. Furthermore, we thank ClipsBnC for study monitoring, CRSCube for data management, BDM Consulting for data analysis and Jeil Pharm for funding this study.